Fabrizio D’Abate, Cristiana Vitale
|
Abstract The ultrasound definition of extracranial carotid artery aneurysms (ECCAs) is unclear. The threshold diameter to use for defining an extracranial carotid artery as aneurysmal is still debated. Similarly, the ultrasound method of choice for measuring the maximum diameter of ECCAs has not been agreed. In this paper we report the case of a patient with a fusiform aneurysm at the level of the carotid artery bifurcation and a large saccular aneurysm of the proximal internal carotid artery, and discuss the information essential to acquire when ECCAs are detected with ultrasound. Keywords: Disclosure: The authors have no conflicts of interest to declare. Received: Accepted: Published online: Correspondence Details: Fabrizio D’Abate, Vascular Laboratory, Vascular Institute, St George’s University Hospital, Blackshaw Rd, Tooting, London SW17 0QT, UK. E: fabrizio.dabate@hotmail.it Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly. |
Extracranial carotid artery aneurysms (ECCAs) are very rare. Overall, ECCAs account for <1% of all arterial aneurysms and for approximately 4% of peripheral artery aneurysms.1,2 The most common aetiologies of ECCAs are atherosclerosis (in 40% of cases) and trauma.1,3 The carotid bulb and the proximal internal carotid artery are commonly affected, whereas ECCAs on the external carotid artery are rare.4 ECCAs can be divided in true and false aneurysms, with the latter being the most common;5 ECCAs can also be classified according to their shape as fusiform or saccular aneurysms.
Many cases of ECCA will remain clinically silent, but in some cases patients will develop symptoms such as pain and/or cranial nerve damage, as well as symptoms related to the compression of adjacent structures.5 The early detection and treatment of ECCAs is of paramount importance, due to the risk of neurological symptoms and rupture. Despite ultrasound being the first-line imaging test used for the detection of ECCAs, there is no generally accepted consensus regarding criteria to define an ECCA.6
Here, we report on a case of a fusiform aneurysm at the level of the carotid artery bifurcation and a large saccular aneurysm of the proximal internal carotid artery. A list of main points to consider when scanning ECCAs is also presented.
Case Presentation
A 62-year-old woman presented with a 10-year history of an enlarging pulsatile left neck mass. She was fit and denied any history of trauma at the level of the neck or any neck surgery or therapy. The patient’s medical history included arterial hypertension and dyslipidaemia, currently being treated with angiotensin-converting enzyme inhibitors and statins.
No other comorbidities or cigarette smoking were reported. There was no history of any neurological symptoms or pain.
On physical examination, the pulsatile mass was non-painful and situated anterior to the left sternocleidomastoid muscle, with no extension to the angle of the mandible. A carotid colour Doppler ultrasound scan was performed with a medium frequency linear array (9–3 MHz) according to the standard protocol, using first a transverse and then a longitudinal view.
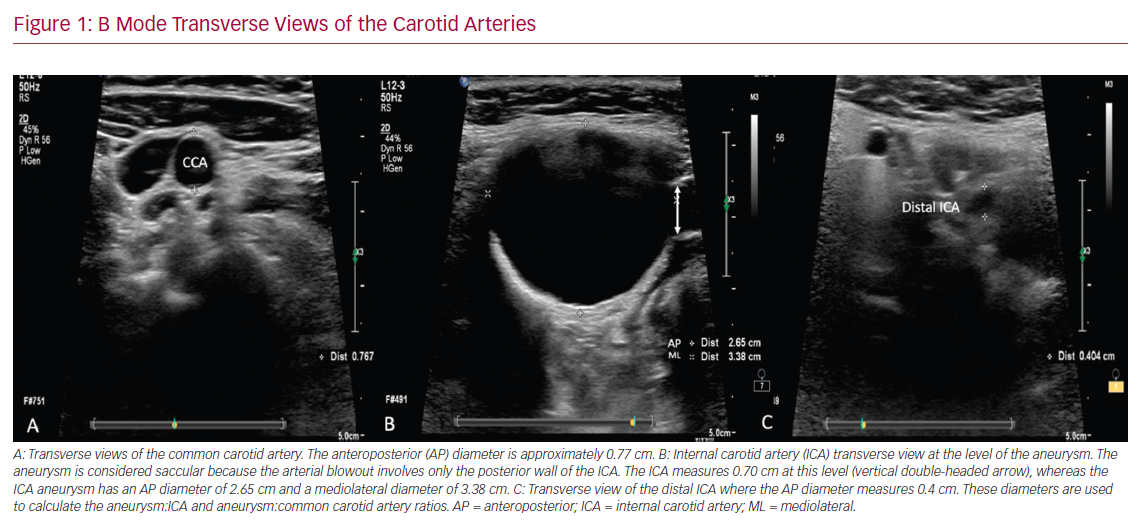
The ultrasound assessment started in B mode for each side at the level of the proximal common carotid artery (CCA) and proceeded upwards to the CCA bifurcation and subsequently to the internal (ICA) and external (ECA) carotid arteries. The B mode overview on the left side showed an abnormal enlargement of the CCA bifurcation and a further enlargement of the proximal ICA. The common carotid bifurcation enlargement involved both the anterior and posterior arterial walls of the CCA bifurcation, suggesting the presence of a fusiform aneurysmal dilatation. In contrast, the enlargement involving the ICA arose from the posterior wall of the vessel only, therefore suggesting either a saccular aneurysm or a pseudoaneurysm of the ICA (Figure 1).
The anteroposterior (AP) and mediolateral (ML) diameters of the arteries were measured at both the level of the maximum enlargement and the level of the normal ICA diameter beyond the aneurysmal segment. The AP diameter of the CCA bifurcation measured 1.8 cm, whereas the AP and ML diameters of the proximal ICA aneurysm measured 2.65 and 3.4 cm, respectively. The AP diameter of the ipsilateral proximal CCA measured 0.77 cm, whereas the native ICA beyond the aneurysmal segment measured 0.4 cm (Figure 1). The lumen of the vessels was patent, with no evidence of intraluminal plaques on B mode, confirmed when colour Doppler flow was applied. The calibre of the ECA and CCA was normal throughout.
B Mode Transverse Views of the Carotid Arteries
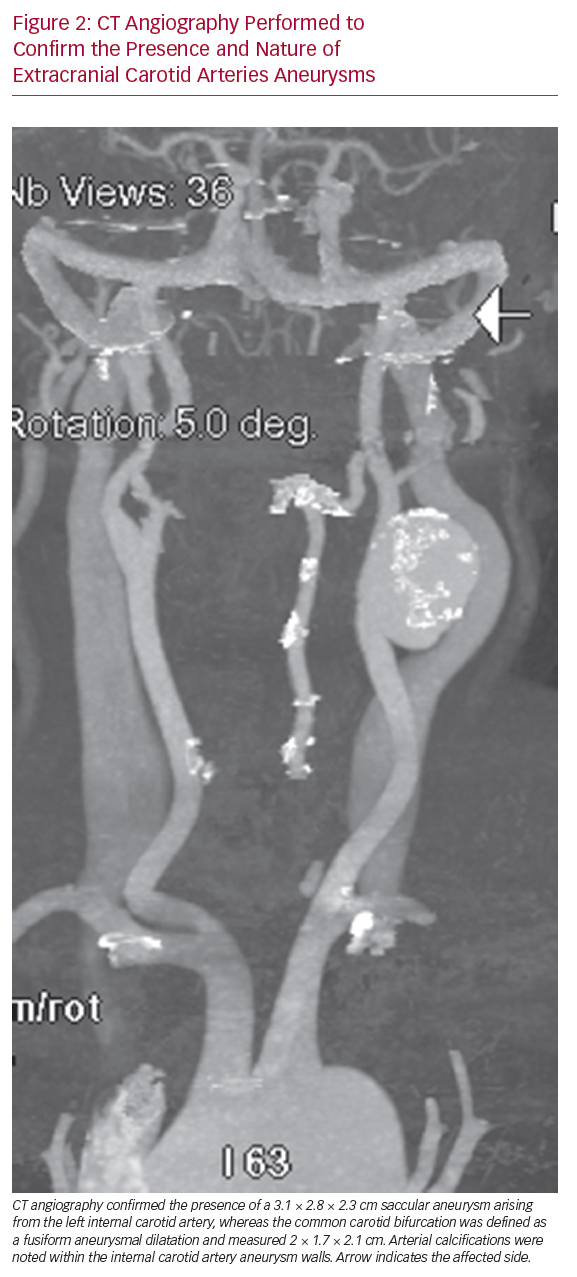
CT angiography (CTA) was subsequently performed to confirm the presence of an ECCA and to determine whether the proximal ICA aneurysm was a true aneurysm or a pseudoaneurysm. The CTA confirmed the presence of a 3.1 cm × 2.8 cm × 2.3 cm saccular aneurysm arising from the left ICA, whereas the CCA bifurcation was defined as a fusiform aneurysmal dilatation that measured 2 cm × 1.7 cm × 2.1 cm (Figure 2). The CTA identified arterial calcifications within the walls of the ICA aneurysm, suggesting that the dilatation was a true aneurysm rather than a pseudoaneurysm.
The patient underwent an elective open repair of the aneurysm with aneurysmectomy and an end-to-end arterial anastomosis between the CCA and ICA with 5–0 prolene. The patient was discharged on day 3 postoperatively, with no complications at follow-up. Histopathological examination of a surgical specimen determined the aneurysm to be atherosclerotic.
Discussion
Although the natural history of ECCAs has not been well defined, their early detection is of importance before ischaemic neurological symptoms appear or the aneurysm ruptures.7,8 Due to its availability and non-invasive nature, ultrasound plays a key role in the detection and characterisation of ECCAs. However, some challenges remain with the ultrasound diagnosis of ECCAs, including unresolved methodological issues.6
Definition of Extracranial Carotid Artery Aneurysms
An aneurysm is defined as a widening of an arterial or venous segment that results from a weakened blood vessel wall. An aneurysm can be present at different levels in the cardiovascular system, and its maximum diameter is often used to monitor its growth until a surgical threshold is reached and an intervention is warranted to prevent its rupture. The cut-off point to define a blood vessel as aneurysmal has been determined for several vascular territories, such as the abdominal aorta, where an AP diameter >3 cm is considered as the cut-off point for the presence of an abdominal aortic aneurysm. Cut-off values to define aneurysms have also been determined for the common iliac arteries and the popliteal arteries, but no such values have been determined for ECCAs. Hence, some authors have defined ECCAs as a localised increase in the calibre of the carotid artery of >50% compared with reference values or the expected vessel diameter.4,9 However, it is important to highlight that due to the scarcity of ultrasound data reporting the mean diameter of normal extracranial carotid arteries, this definition has intrinsic limitations. One study reported normal mean (±SD) diameters of 4.66 ± 0.78 and 6.10 ± 0.80 mm for the ICA and CCA, respectively, in women, and 5.11 ± 0.87 and 6.52 ± 0.98 mm, respectively, in men.10 Another study reported that the diameter of the CCA lumen ranged from 4.3 to 7.7 mm.11
However, considering that there are no significant differences in lumen diameter between the left and right carotid arteries and that ECCAs are usually unilateral, the contralateral normal side can be used as the reference value to determine the normal expected diameter of the vessel.12 This may prove to be particularly useful in the presence of a dilated or aneurysmal carotid bulb, which, by definition, is an already dilated segment. Another definition, proposed by De Jong et al., for ECCAs at the bifurcation level is the presence of bulb dilatation >200% of the diameter of the ICA or 150% of the diameter of the CCA, whereas, for ECCAs at the level of the distal extracranial ICAs, it is the presence of a dilatation >120% of the diameter of the normal ipsilateral ICA.13
In the present case report, the maximum AP diameter of the ICA aneurysm was 562% of the AP diameter of the non-aneurysmal ICA and 244% larger than the AP diameter of the non-aneurysmal CCA. According to the ECCA definition suggested by De Jong et al.,13 the AP diameter of the carotid bifurcation was 350% larger than the non-aneurysmal ipsilateral distal ICA AP diameter and 134% larger than the CCA AP diameter, thus suggesting the presence of a carotid bifurcation aneurysm.
Measurement of Extracranial Carotid Artery Aneurysm Diameter and Practical Ultrasound Considerations
Depending on the ultrasound measurement technique used to evaluate the diameter at the level of abdominal aortic aneurysms, there are three methods that can be used to measure artery diameter: outer to outer, inner to inner or leading edge to leading edge (Figure 3). The diameters are usually measured using a B mode image and both transverse and longitudinal views can be used. The anterior and posterior arterial walls (AP diameter) or the medial and lateral arterial walls (ML diameter) are usually measured. The measurement of the ML diameter of a blood vessel is typically less precise than that of the AP diameter. This is due to poor lateral resolution of the medial and lateral wall boundaries, which are parallel to the ultrasound beam, thus producing poor images. Therefore, ML diameter measurements are more prone to error and less reproducible. If the ML diameter is the largest diameter of the aneurysm, a different angle of insonation should be used in an attempt to improve the lateral resolution of the wall boundaries.
CT Angiography Performed to Confirm the Presence
Different Methods That Can be Used to Measure Aortic Diameter
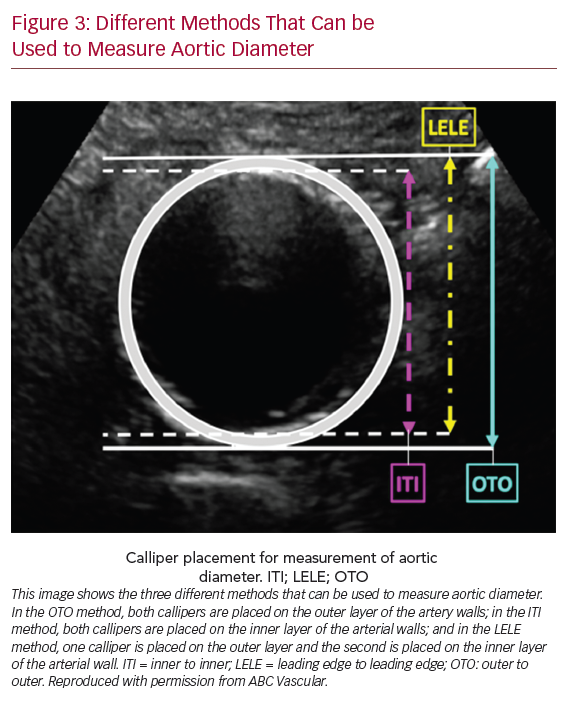
We also suggest measuring the maximum diameter using a transverse view. However, in the case of a saccular aneurysm or in the presence of tortuous aneurysm, the longitudinal view can be of help in defining the maximum diameter. To obtain the maximum diameter of a vessel or aneurysm, the ultrasound beam must be perpendicular to its long axis; however, often in presence of a tortuous vessel, the ultrasound beam is horizontal to the actual vessel, therefore yielding a larger than actual vessel diameter. Confirming the diameter measurements obtained from the transverse view with the measurements obtained from the longitudinal view can improve the degree of confidence in the measurement recorded. Because a gold standard ultrasound method to measure the largest diameter of ECCAs is lacking, we also suggest that the operator should report the methodology used to determine the maximum diameter of the enlarged vessel.
Location of Extracranial Carotid Artery Aneurysms
Depending on the location of the aneurysm, ECCAs can be classified into five types.4,8 Because the surgical options vary depending on this classification, it is important to describe the location of the ECCAs in the ultrasound report. When ultrasound views of the aneurysm are limited by patient body habitus of the depth of vessels, the use of a low-frequency curvilinear array (2–9 MHz) may improve the visualisation of the extension of the ECCAs and enable better depiction of the location of the aneurysm and its correlation with other anatomical structures. ECCAs represent 1% of all peripheral aneurysms. To date, there is no consensus as to whether patients with an ECCA should be screened for the presence of other aneurysms. Because patients with an aneurysm have a higher risk of having an aneurysm in a different location, it may be good practice to always search for peripheral aneurysms in patients with an ECCA.
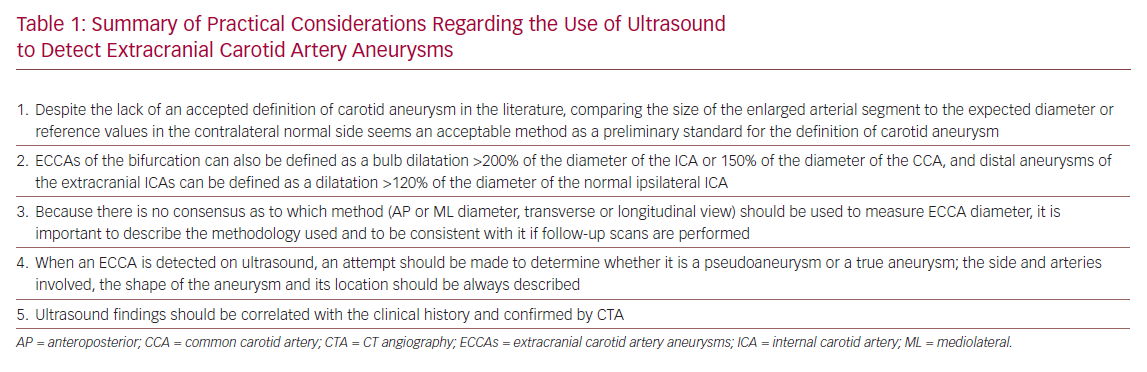
Summary of Practical Considerations Regarding
ECCAs can be divided in true aneurysms and pseudoaneurysms. True aneurysms, mostly caused by atherosclerosis, are localised enlargements of the artery diameter, with integrity of all three vascular layers, whereas pseudoaneurysms occur in presence of an interruption of the continuity of all three layers of the arterial wall. Most pseudoaneurysms develop as a complication of carotid endarterectomy or cervical trauma, such as blunt injury or penetrating trauma.5
A true aneurysm can be classified as either a fusiform aneurysm (the most common type, in which the whole cross-sectional area of an arterial segment is enlarged) or a saccular aneurysm (in which the dilatation affects only one side of the arterial segment). The main challenge with ultrasound is distinguishing a true saccular aneurysm from a pseudoaneurysm, especially when ECCAs are localised in the more distal regions of the ICA.
As shown in this case report, despite the ultrasound being able to identify the presence of a proximal ICA dilatation, it was unclear whether this dilatation was a true saccular aneurysm or a pseudoaneurysm, because the dilatation was affecting only the posterior aspect of the ICA wall. The CTA suggested that this was a true saccular aneurysm based on the presence of arterial wall calcifications, which would suggest integrity of the arterial wall. In addition, using a high-definition B mode image one could note the intima and media layers throughout the aneurysm, thus suggesting the presence of a true aneurysm.
It is always important to correlate the ultrasound findings to the clinical history of the patient, because this could provide clues as to the nature of the enlargement. In the present case, there was no history of trauma or procedures to the neck region.
Differential Diagnoses
There are other conditions that may mimic the presence of a carotid aneurysm. Coiling and/or kinking of the carotid arteries, dilatation of the internal jugular vein, bulb ectasia and carotid body tumour, lymph nodes, neck tumours and peritonsillar abscesses are the main differential diagnoses to be considered during an ultrasound assessment of ECCAs.
Conclusion
Despite the lack of an accepted definition of aneurysm at the level of the carotid arteries in the literature, comparing the size of the enlarged arterial segment to the expected diameter or to reference values in the contralateral normal side seems an acceptable method to define a carotid aneurysm. When measuring an ECCA it is important to describe the methodology used and to use it consistently if follow-up scans are performed. Characteristics of the carotid aneurysm, such as the side and arteries involved, the shape of the aneurysm and its location, should be always described. An attempt to distinguish a pseudoaneurysm from a true aneurysm should be made when ECCAs are detected. Ultrasound findings should always be correlated to the patient’s clinical history and confirmed by CTA. The main points discussed in this case report are summarised in Table 1 and clearly show the need to develop a consensus regarding the methodology to use to assess ECCAs.
- El-Sabrout R, Cooley DA. Extracranial carotid artery aneurysms: Texas Heart Institute experience. J Vasc Surg 2000;31:702–12.
Crossref| PubMed - McCollum CH, Wheeler WG, Noon GP, DeBakey ME. Aneurysms of the extracranial carotid artery. Twenty-one years’ experience. Am J Surg 1979;137:196–200.
Crossref| PubMed - Rosset E, Albertini JN, Magnan PE, et al. Surgical treatment of extracranial internal carotid artery aneurysms. J Vasc Surg 2000;31:713–23.
Crossref| PubMed - Attigah N, Külkens S, Zausig N, et al. Surgical therapy of extracranial carotid artery aneurysms: long-term results over a 24-year period. Eur J Vasc Endovasc Surg 2009;37:127–33.
Crossref| PubMed - Longo GM, Kibbe MR. Aneurysms of the carotid artery. Semin Vasc Surg 2005;18:178–83.
Crossref| PubMed - Welleweerd JC, den Ruijter HM, Nelissen BG, et al. Management of extracranial carotid artery aneurysm. Eur J Vasc Endovasc Surg 2015;50:141–7.
Crossref| PubMed - Bemelman M, Donker DN, Ackerstaff RG, Moll FL. Bilateral extracranial aneurysm of the internal carotid artery – a case report. Vasc Surg 2001; 35:225–8.
Crossref| PubMed - Zhang Q, Duan ZQ, Xin SJ, et al. Management of extracranial artery aneurysms: 17 years’ experience. Eur J Vasc Endovasc Surg 1999;18:162–5.
Crossref| PubMed - Rosset E, Albertini JN, Magnan PE, et al. Surgical treatment of extracranial internal carotid artery aneurysms. J Vasc Surg 2000;31:713–23.
Crossref| PubMed - Limbu YR, Gurung G, Malla R. Assessment of carotid artery dimensions by ultrasound in non-smoker healthy adults of both sexes. Nepal Med Coll J 2006;8:200–3.
PubMed - Krejza J, Arkuszewski M, Kasner SE, et al. Carotid artery diameter in men and women and the relation to body and neck size. Stroke 2006;37:1103–5.
Crossref| PubMed - Williams MA, Nicolaides AN. Predicting the normal dimensions of the internal and external carotid arteries from the diameter of the common carotid. Eur J Vasc Surg 1987;1:91–6.
Crossref| PubMed - de Jong KP, Zondervan PE, van Urk H. Extracranial carotid artery aneurysms. Eur J Vasc Surg 1989;3:557–62.
Crossref| PubMed
