Roger Barranco Pons, Isabel Rodriguez Caamaño, Marta de Dios Lascuevas
|
Abstract Transradial access (TRA) has become the standard approach for cardiac intervention, with a large body of evidence demonstrating a lower incidence of vascular complications, better patient experience and cost reduction. There has been increasing interest in using TRA both for diagnostic neuroangiography and for interventional neurovascular procedures. The aim of this article is to discuss the advantages and limitations of TRA for neurointerventions. General technical details, such as pre-procedure recommendations, prevention of spasm and occlusion, haemostasis protocols and distal TRA puncture, are also described, along with the specific technical details of TRA for aneurysm embolisation, stroke thrombectomy and other neurovascular interventions. TRA provides additional tools to the neurointerventionist and – with appropriate training – the whole spectrum of intervention procedures can be achieved using this approach. Keywords: Radial access, neurointerventions, stroke, brain aneurysm embolisation Disclosure: The authors have no conflicts of interest to declare. Received: Accepted: Published online: Correspondence Details: Roger Barranco Pons, Hospital Universitari de Bellvitge, Feixa llarga s/n. 08907, Hospitalet de Llobregat, Barcelona, Spain. E: rbarranco@bellvitgehospital.cat Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly. |
Percutaneous radial access was described by Campeau in 1989 for coronary angiography in 100 patients.2 Since then, a growing body of evidence suggests that TRA is safer for patients and more cost-effective compared with TFA. Radial access reduces mortality and major adverse cardiovascular events and improves safety, with reductions in major bleeding and vascular complications across the whole spectrum of patients with coronary artery disease.3 The 2018 European Society of Cardiology/European Association for Cardio-Thoracic Surgery guidelines on myocardial revascularisation state that radial access is preferred for any percutaneous coronary intervention irrespective of clinical presentation, unless there are overriding procedural considerations.4
Matsumoto and colleagues described TRA for neurointervention procedures in 2000 and there are large series showing the safety and feasibility of transradial cerebral angiography.5–9 Furthermore, complex interventions for both ischaemic and haemorrhagic disease of the posterior and anterior circulation have been reported, highlighting the feasibility of this approach despite the use of femoral-designed devices.10–16 Adoption of TRA has been slow in the neuroendovascular field, although interest has increased in recent years.
In this review, we examine the advantages, limitations and technical details of neurovascular interventions using TRA. Specific technical details for neurointerventions in haemorrhagic and ischaemic disease are also described.
Advantages
The vast experience of using TRA in cardiology has yielded considerable knowledge about its safety, although some specific conditions regarding neurointerventions need to be considered. Because of its recognised safety, TRA might be the first option in patients with severe femoral or aorto-iliac disease, obese patients with deep femoral arteries or patients with high haemorrhagic risk. Anticoagulants do not need to be withdrawn when using TRA in patients receiving these medications. Even though it is not well established that TRA is necessarily safer in patients with atherosclerotic disease involving the aortic arch for neuroendovascular procedures, it is known that in the thoracic region calcifications are more often formed in the aortic arch and descending rather than ascending aorta.17,18 For some TFA-challenging anatomies such as a bovine or type III aortic arch type, TRA might be a better option. TRA also has the advantage of offering direct access to the vertebrobasilar system. Other TRA access advantages include better tolerability and short haemostasis times allowing for very short hospital stays – both in diagnostic angiographies and scheduled interventions. From a financial perspective, there are savings in access complication costs, closure devices and hospital stay.19
Limitations, Crossovers and Complications
Despite the feasibility and known advantages of TRA, there are access site and cerebrovascular-specific limitations to TRA. In the field of cardiology, recent registries have shown that risk of access failure and conversion to TFA has a low conversion index (1.5%), due to operator experience, improved techniques and material.20,21 This contrasts with older trials, in which the failure index was 7.3% compared to 2% for TFA.22
There are data showing that experience performing approximately 30–50 TRA cerebral angiograms is needed to become comfortable with TRA. During the learning curve, there is a reduction in crossover rate and fluoroscopy times, with better success in catheterising all intended supra-aortic arteries.23,24 In a recent systematic review of 1,342 procedures of TRA for neurointerventions, the crossover rate to TFA was 4.77%. Among the crossover group, 10.93% crossed over because of the failure to obtain radial artery (RA) access and because of the inability to catheterise the target vessel in 89.06%.25
The two most common complications associated with TRA are RA spasm and RA occlusion. RA spasm is noted in 15–30% of cases, but this can be reduced to 6–10% with intra-arterial administration of nitroglycerin and a calcium channel blocker.26,27 RA occlusions have been reported to occur at rates of 0.8–33% in different series, but this can be reduced significantly with precautions described in the following section.20,28 Furthermore, RA occlusion is clinically silent in the majority of cases, secondary to collateral circulation via the palmar arch. Apart from difficulty using the same RA for future access, the clinical implications of RA occlusion are very limited.29,30
Clinically relevant complications, such as hand ischaemia requiring amputation and compartment syndrome, have been reported, but are exceedingly rare.31 Minor complications, such as extended access site pain, haematoma and bruises, are other possibilities.32 There are some anatomic variations of the RA that operators should be aware of, such as high brachial artery bifurcation, radial or brachial artery loops, tortuosity of the RA and the presence of an accessory RA.
For cerebrovascular angiography specifically, some challenging anatomies may cause difficulty in catheterisation of the vessels. These include the left vertebral artery, a proximal right common carotid artery (CCA) with an acute angle or a loop in the left CCA. Another anatomic variant, subclavia lusoria (reported to be present in 0.1–0.8% of cases) may present some catheterisation difficulties from a right TRA.33
Technical Details
Pre-procedure
Traditionally, assessment of the collateral circulation to the hand via Allen’s test and the Barbeau test (objective Allen’s test using pulse oximetry and plethysmography) has been used prior to TRA. However, significant controversy exists regarding the need for pre-procedural collateral circulation testing.34
Of note, the Minimizing Adverse Haemorrhagic Events by TRansradial Access Site and Systemic Implementation of angioX (MATRIX) trial randomised more than 4,000 patients to TRA (regardless of the pre-procedure collateral testing result) and found no post-procedure symptomatic hand ischaemia.35
Size Matters
The RA – having a smaller calibre – has a series of limitations regarding the diameter of the materials used and, in some patients, this may preclude its use.36 In our experience, the use of ultrasound both for puncture and to measure the diameter of the artery serves to correctly select and rule out radial procedures in patients in whom the radial inner diameter is <1.5 mm (Figure 1). It is important to note that when measuring the diameter of the artery there are factors that can influence the results (check that the patient is not cold and is calm).
Several authors have analysed the diameter of the RA with ultrasound and correlated the risk of occlusion depending on the external diameter of the introducer used.21 They found no correlation of the radial diameter with BMI.22 A cannulation procedure can still be attempted with smaller radial diameters, but the chances of vasospasm and TFA conversion are higher, especially in young women.37 Administration of topical lidocaine and nitroglycerin or subcutaneously administered nitroglycerin prior to puncture has been shown prospectively to increase the diameter of the RA and facilitate TRA.34,38,39
In most patients, performing a diagnostic angiogram with a 5 Fr introducer will be possible. A cross-sectional inner RA diameter of approximately 1.5–2.0 mm is required for a 5 Fr sheath and diagnostic catheters. Some interventions can be done through a 6 Fr or 7 Fr thin-walled specific radial sheath, from which a 6 Fr (0.070 inner diameter [ID] system) or 7 Fr guiding catheter can be used. For a 6 Fr sheath, we recommend at least 1.9 mm of radial diameter. The Terumo slender 6 Fr and Prelude Ideal (Merit Medical) thin-walled specific radial sheaths have an outer diameter of 2.44 mm compared to other non-thin-walled radial sheaths (2.63−2.8 mm) and Terumo 6 Fr femoral sheaths (2.62 mm). Other interventions may require larger sheaths and intermediate catheters for support. In our institution, for cases in which large-bore sheaths (0.088 ID) are needed, this approach is used if the radial diameter is at least 2.3 mm.
Prevention of Spasm and Occlusion
While essentially clinically silent, prevention of RA occlusion is important, especially with regard to consideration of further procedures. RA occlusion rates have been shown to increase with increasing sheath diameter, especially when the outer diameter of the sheath exceeds the inner diameter of the RA. The use of sheathless TRA has been described as allowing for larger ID guide catheters to be placed without an attendant increase in outer diameter from sheath placement.40
Administration of unfractionated heparin at therapeutic levels (50 IU/kg or 5,000 IU) has been shown prospectively to lead to a sixfold reduction in RA occlusion rates, with higher rates of administration (100 IU/kg) further decreasing the incidence of RA occlusion.41,42 However, the best route for heparin administration remains unclear, with no difference between intravenous and intra-arterial bolus administration through a sheath with regard to RA occlusion rates.41–43
The administration of intra-arterial antispasmodic medications has been shown prospectively in multiple trials to reduce RA spasm although without a clear consensus on the most effective combination and dose.44–46 A meta-analysis of 22 randomised trials found the lowest rates of RA spasm following intra-arterial administration of nitroglycerin 200 µg and verapamil 5 mg.47 We recommend preparation and administration of a cocktail in a 20 ml syringe with 200 µg nitroglycerin, 5 mg of verapamil and 4,000 IU heparin. Once the sheath is in place, we aspirate blood to fill the 20 ml cocktail syringe and inject it gently to minimise discomfort. Recalcitrant RA spasm can be managed with further administration of antispasmodic medications.
Radial Access, Step-by-step
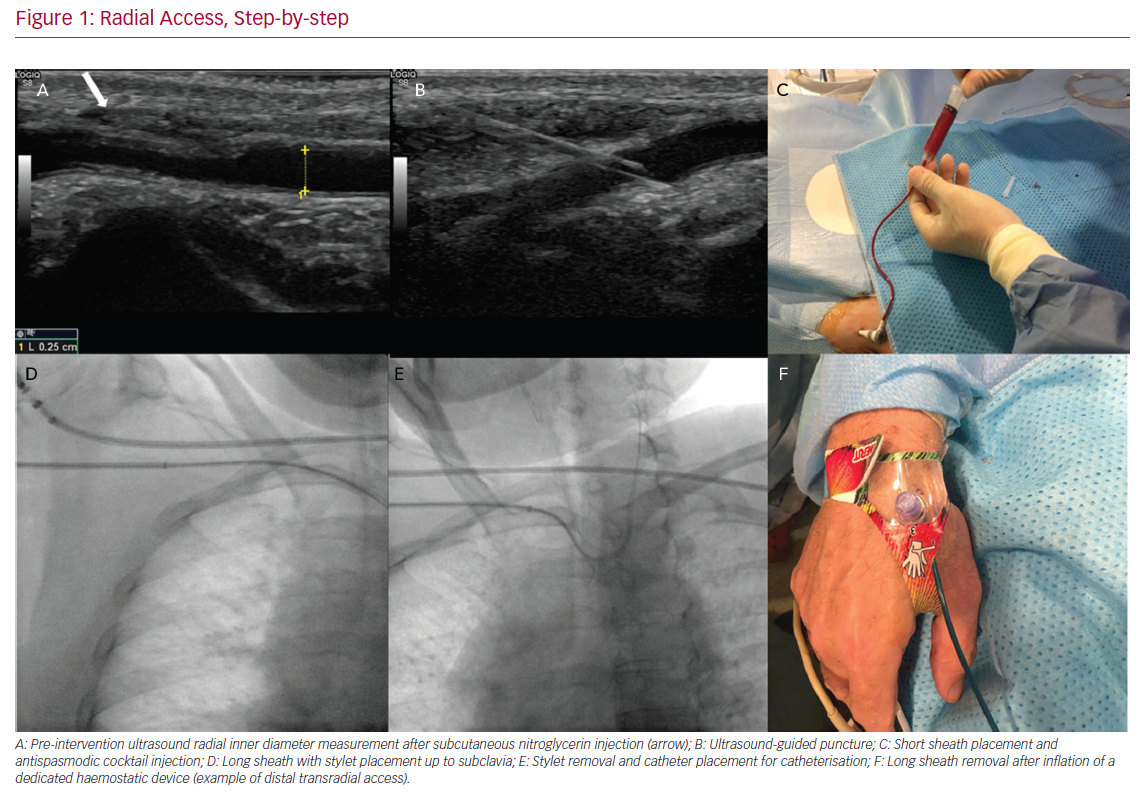
The use of a patent haemostasis technique significantly improves RA patency rates. Patent haemostasis has been shown to reduce rates of RA occlusion by 75% compared with conventional pressure application, either manually or with a compressive haemostatic band.48 Prophylactic ulnar artery compression, added to patent haemostasis, is reported to result in RA occlusion rates <1%.49 Although use of patent haemostasis is paramount in preventing RA occlusion, there is no consensus on the optimal protocol for deflation of the radial haemostatic band.48 If RA occlusion is encountered immediately post-operatively, ulnar compression, as well as administration of low-molecular-weight heparin can promote recanalisation.50
Distal Transradial Access
Recently, distal transradial access (dTRA) has received more attention in the attempt to improve some of the limitations of conventional radial access.51 Recent series have reported both cerebral angiography and neurointerventions successfully performed through dTRA.52,53 In puncturing the RA in the anatomic snuffbox, distal to the origin of superficial palmar branch, in the case of occlusion there is a theoretically lower risk of compromising the superficial palmar arch and less risk of hand ischaemia. In addition, the forearm can be kept in mid-prone position right next to the body, which reduces discomfort because the position is more ergonomic. In interventions in which left RA is needed, it is very difficult to use the left conventional radial approach as most neuro suites are designed (e.g., the position of controls and the screen) to work on the right side of the patient. In these cases, left distal radial access is particularly useful to perform left vertebral artery procedures via the left forearm. The left forearm is kept partially flexed over the patient’s abdomen with the hand close to the left groin and then taped in place (Figure 2).
The diameter of the distal RA, which can be smaller in the snuffbox compared with the conventional radial puncture site therefore predisposing to spasm, may result in higher conversion rates.54 We strictly recommend the use of ultrasound to measure, select, and puncture the distal RA at the snuffbox.
Another advantage of puncturing the dTRA is that patency rates are reported to be very high and this permits short haemostatic protocols.55 In addition, in the case of artery occlusion, this normally happens at the level of the snuffbox. Therefore, the RA can still be punctured in the forearm.
Haemorrhagic Disease
Because haemorrhagic disease can involve both acute ruptured disease or elective cases, we recommend first trying radial access in elective rather than acute cases. During this kind of intervention – in which larger diameter catheters and sheaths might be used – sedation and general anaesthesia also help to reduce the incidence of RA spasm by reducing both anxiety and sympathetic drive.56,57
Aneurysm Treatment
A 6 Fr short sheath can be used to introduce a 0.070 ID system after spasmolytics are administered. The 0.070 ID system can either be a Simmons-2 guiding catheter Envoy (Codman Neuro) or any other guiding catheter using a long Simmons-2 catheter or using a wire exchange. In the event that simple coiling is used, a 6 Fr catheter such as Envoy DA may allow the use of a 0.058 intermediate catheter if needed (e.g. a 5 Fr Navien [Medtronic] or 5 Fr Sofia [Microvention]). Most aneurysm embolisation techniques can be performed through
a 6 Fr guiding catheter, which allows two microcatheters in order to perform balloon-assisted coiling (BAC) and/or stent-assisted coiling (SAC). In more challenging cases where greater support is needed, larger sheaths and intermediate catheters may be required.
Left Distal Transradial Access for Flow Diverter Placement
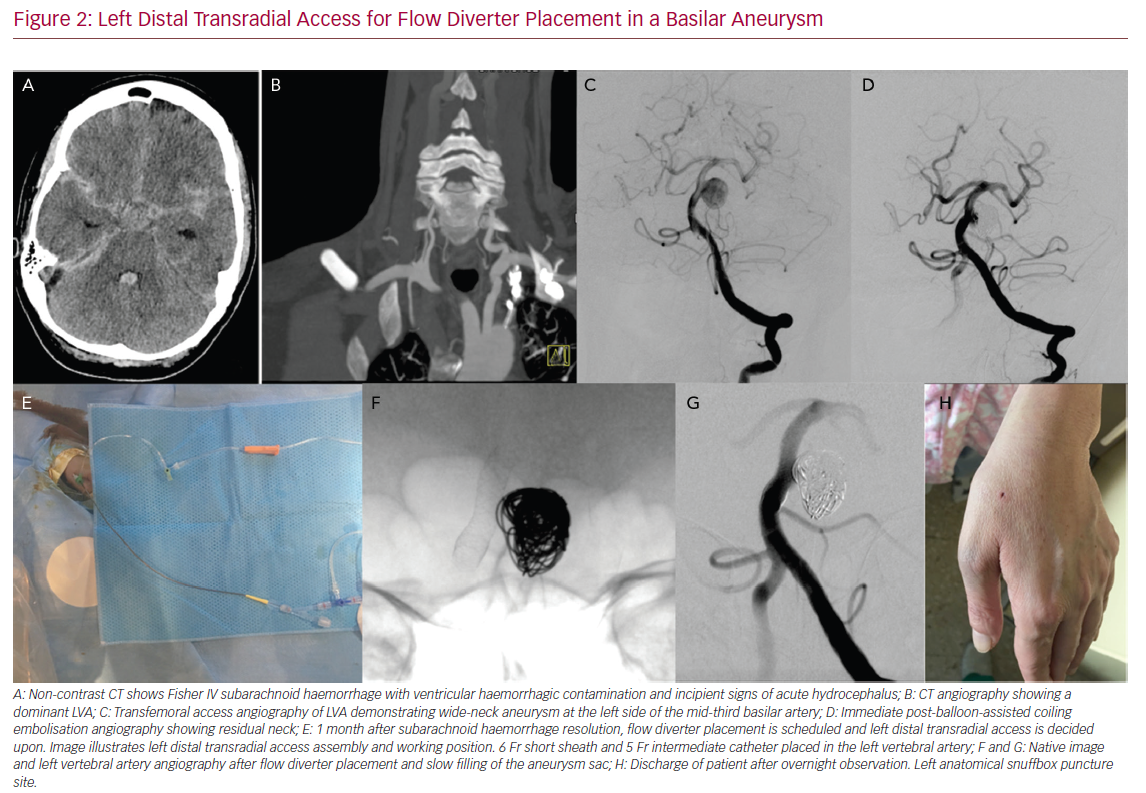
In some embolisation cases where a double access is needed to control both carotids, both vertebral arteries, a posterior and anterior circulation artery, or to do a transcirculation approach, TRA can be also useful.58 Access can be through both RAs or combining TRA and TFA. In cases with ruptured aneurysms, we tend to administer the radial cocktail without heparin and give systemic heparin once the first coil is detached and BAC is performed. The decision as to when to give heparin depends more on the need for neurovascular intervention and safety rather than prevention of RA occlusion. In our experience, the use of radial access for elective aneurysm embolisation also allows early patient discharge after overnight observation.
Treatment of Arteriovenous Malformation and Arteriovenous Fistula
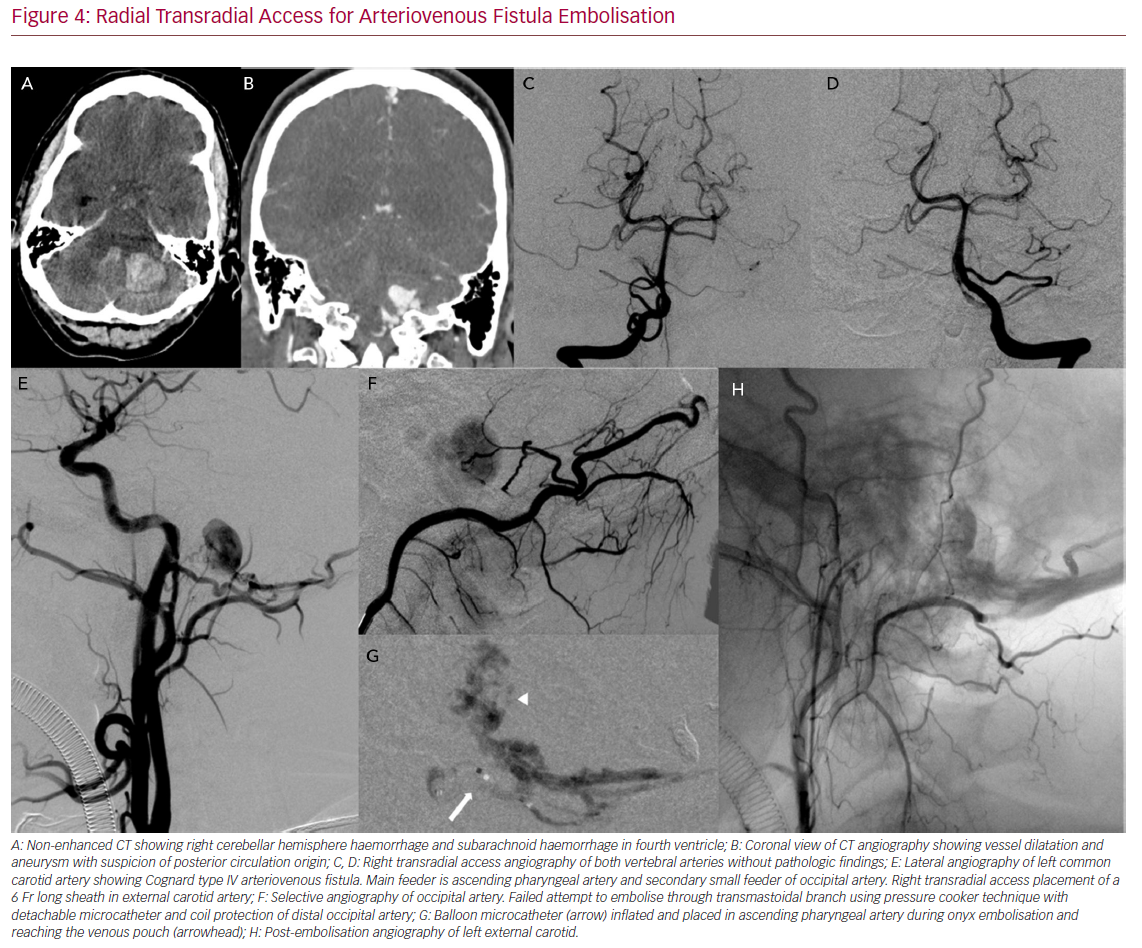
Complex interventions such as arteriovenous malformation (AVM) and arteriovenous fistula (AVF) embolisation can also be done through TRA (Figure 4). In cases of a multiple feeder AVF or AVM, double access may be required. While one access can be the treating one, in other vessels diagnostic catheters may help to ensure all the feeders are closed. TRA can be used as the main treating access, as a control catheter access or as a multiple access from both radial arteries (for the left radial a left dTRA is recommended).
Most embolisation techniques, such as single microcatheter, balloon assisted embolisation, pressure cooker technique with an Echelon-10 (Microvention), and detachable tip microcatheter can be applied through a 6 Fr guiding catheter and a 6 Fr radial sheath.59 When the pressure cooker technique with magic catheter is required, a 6 Fr long sheath or a 7 Fr guiding catheter through a 7 Fr radial sheath can be used.59
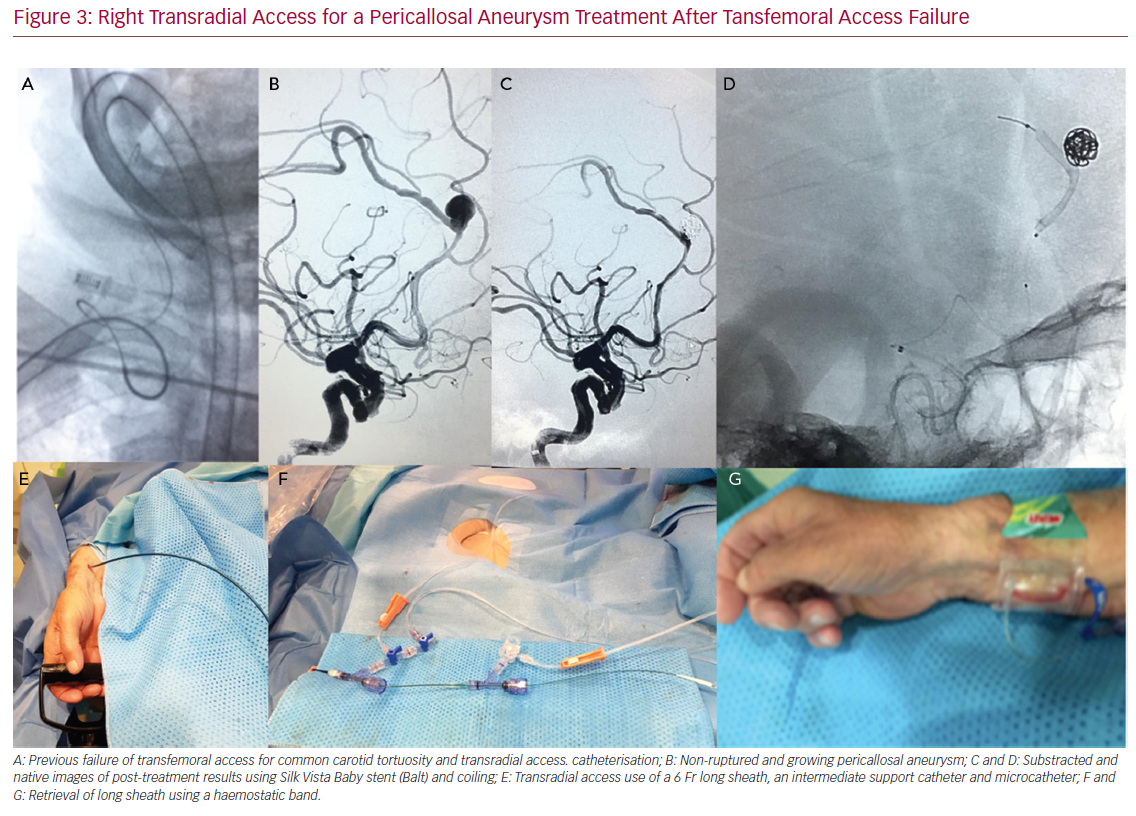
Right Transradial Access for a Pericallosal Aneurysm
Ischaemic Disease
Stroke Thrombectomy
As is commonly recognised, stroke revascularisation has a crucial difference compared with other neurovascular procedures in that not wasting time is mandatory.
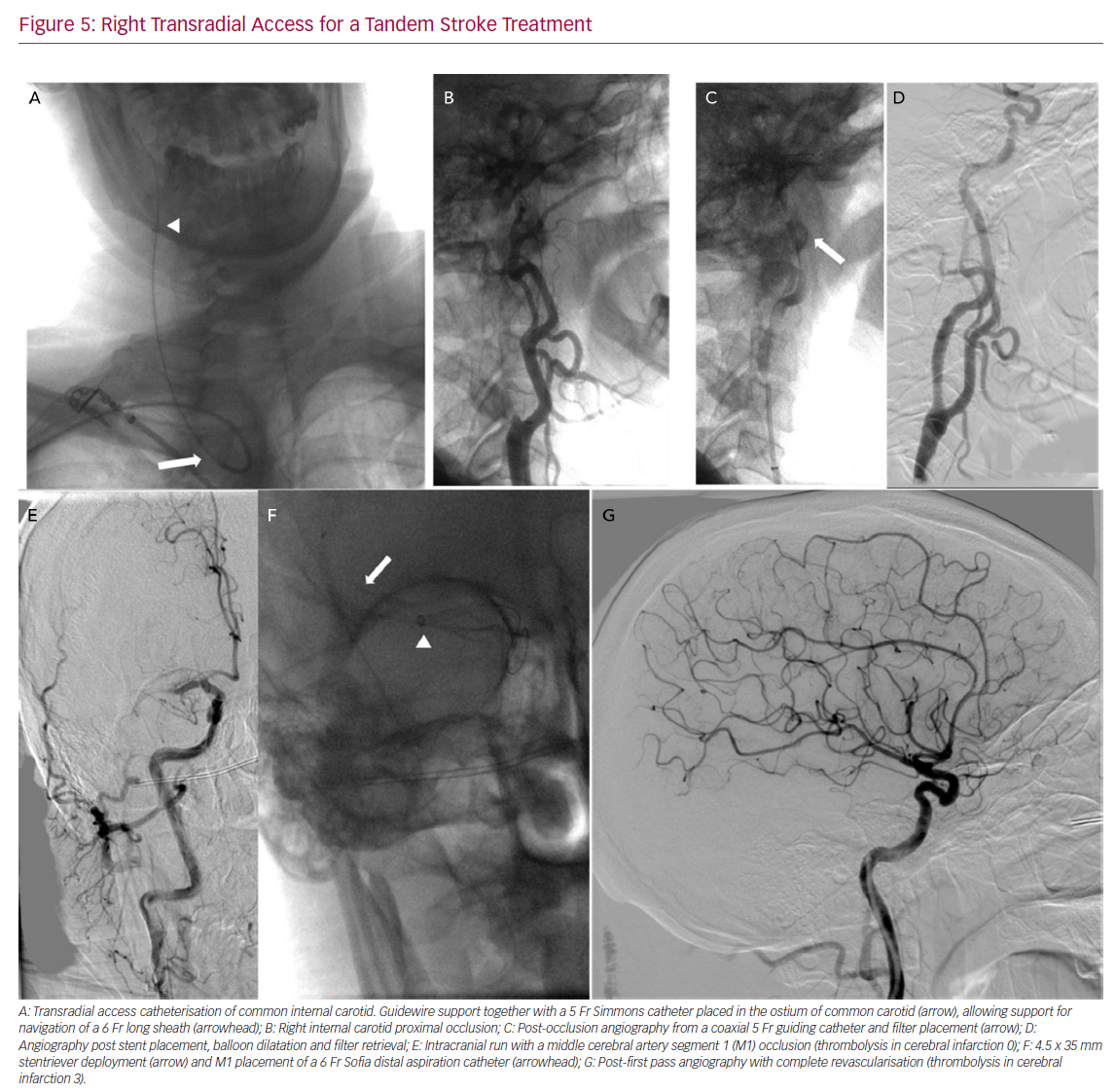
In our experience, we have performed TRA thrombectomies once both the operator and the rest of the team have gained enough experience performing diagnostic angiographies and other interventions. Even with experience, the first TRA cases were done after TFA failure. With experience, cases can be selected to do TRA as a first approach in vertebro-basilar stroke, bovine arch and type III aortic arch. One potential advantage of TRA over TFA is that the smaller radial and brachial diameter acts as a support to the system when dealing with vascular tortuosity. Some authors have compared TRA and TFA performance in stroke thrombectomy in different challenging aortic anatomies,60 demonstrating its feasibility (Figure 5).
Before proceeding with a TRA we first measure the RA inner diameter and rule the procedure out if the diameter is <2.3 mm in order to minimise the risk of RA spasm. The simplest means of performing the thrombectomy is with a stentriever while aspirating from a 6 Fr guiding catheter placed in the ICA through a 6 Fr specific radial sheath. Either a Simmons-2 shaped 6 Fr Envoy guiding catheter placed directly, or a 6 Fr guide catheter coaxially navigated into the target ICA over a 125 cm Simmons-2 shaped diagnostic catheter can be used. However, this technique means not using a distal aspiration catheter as a proximal occlusion balloon.10
For anterior circulation stroke, in the case of the Direct Aspiration First Pass Technique (ADAPT) or distal aspiration with retrievable stent assisted thrombectomy, a large 6 Fr sheath is required.
As previously described, a 6 Fr large sheath can placed in the ICA, having enough support through the arch and allowing aspiration catheters up to 0.71 inches, such as Penumbra ACE 6 (Penumbra) Sofia 6 Fr+, React 071 (Medtronic), or AXS Vecta 71 (Stryker). Having a 6 Fr long sheath in place also allows treatment of tandem stroke, and, if required, carotid stents can be place through it.
For operators who prefer using balloon occlusion aspiration, there are several options:
- When the radial diameter is small, a 7 Fr radial thin-walled sheath allows a Cello 6+ balloon guide catheter (Medtronic).
- Once a Simmons catheter is introduced into the 7 Fr sheath and used to navigate to the target ICA over an exchange guidewire, the catheter is removed and the Cello balloon guide catheter is advanced. One possible disadvantage of this technique is that as the balloon inner diameter is smaller there is a theoretically greater risk of catheter occlusion in cases with high thrombotic burden.
- When in the pre-procedural ultrasound radial diameter is at least 2.4 mm, an 8 Fr balloon guide catheter can be used.
Radial Transradial Access for Arteriovenous Fistula Embolisation
More data are needed regarding the safety of prophylactic heparin administration to prevent RA occlusion owing to the risk of haemorrhagic conversion after stroke. Most centres perform thrombectomy without systemic heparinisation, even though some heparin is infused through the saline perfusion of the catheters.
In our experience, in those cases with bridging therapy and previous recombinant tissue plasminogen activator, we do not administer heparin to prevent RA occlusion. In other cases, once the procedure is finished without complications, we administer lower heparin doses (2,000 IU) just before retrieving the sheath from the RA.
Carotid Stenting
Access site bleeding and vascular access complications are the most common adverse events after carotid artery stenting (CAS) with TFA. The need for transfusion may significantly increase the stroke risk as well.61 Complex aortic arch is a risk factor for technical failures, and type III aortic arch with friable atheromas is the most risky feature for CAS complications.62 The highest prevalence of atherosclerosis distribution is in the descending aorta (38.2%), followed by arch (27.6%) distal to the innominate artery, especially with increasing age.63 On the other hand, symptomatic stroke (14%) contralateral to the treated carotid stenosis indicates that aortic arch catheter manipulation is a cause of atheroembolic brain lesion.61,64 TRA may minimise catheter contact in the arch, particularly for right ICA and left bovine ICA. Transradial CAS can be successfully performed by experienced operators with a low complication rate in a large percentage of patients.65,66
From a technical point of view, in terms of the type of carotid stent and the required ID of the delivery system, a 6 Fr or 7 Fr guiding catheter may be used. When stents require a larger delivery system or better support is needed, a long 5 Fr or 6 Fr sheath may also be used. Either way, a distal protection filter system can also be used. If a proximal balloon occlusion technique is desired, it also can be done as described above.
Right Transradial Access for a Tandem Stroke Treatment
TRA has become the standard approach for cardiac intervention due to the large body of evidence demonstrating the lower incidence of vascular complications, better patient experience and cost reduction. The neurovascular field can benefit from the available knowledge from the cardiology field. TRA provides additional tools for the neurointerventionalist and, with adequate training, the whole spectrum of intervention procedures can be carried out. The use of ultrasound is recommended to measure and puncture the RA, as well as to begin the learning curve through performing diagnostic angiography.
- Kaufmann TJ, Huston 3rd J, Mandrekar J, et al. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 2007;243:812–9.
Crossref| PubMed - Campeau L. Percutaneous radial artery approach for coronary angiography. Cathet Cardiovasc Diagn 1989;16:3–7.
Crossref| PubMed - Snelling BM, Sur S, Shah SS, et al. Transradial access: lessons learned from cardiology. J Neurointerv Surg 2018;10:487–92.
Crossref| PubMed - Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165.
Crossref| PubMed - Matsumoto Y, Hokama M, Nagashima H, et al. Transradial approach for selective cerebral angiography: technical note. Neurol Res 2000;22:605–8.
Crossref| PubMed - Park JH, Kim DY, Kim JW, et al. Efficacy of transradial cerebral angiography in the elderly. J Korean Neurosurg Soc 2013;53:213–7.
Crossref| PubMed - Brunet MC, Chen SH, Sur S, et al. Distal transradial access in the anatomical snuffbox for diagnostic cerebral angiography. J Neurointerv Surg 2019;11:710–3.
Crossref| PubMed - Snelling BM, Sur S, Shah SS, et al. Transradial cerebral angiography: techniques and outcomes. J Neurointerv Surg 2018;10:874–81.
Crossref| PubMed - Jo KW, Park SM, Kim SD, et al. Is transradial cerebral angiography feasible and safe? A single center’s experience. J Korean Neurosurg Soc 2010;47:332–7.
Crossref| PubMed - Sur S, Snelling B, Khandelwal P, Caplan JM. Transradial approach for mechanical thrombectomy in anterior circulation large-vessel occlusion. Neurosurg Focus 2017;42:1–4.
Crossref| PubMed - Schönholz C, Nanda A, Rodriguez J, et al. Transradial approach to coil embolization of an intracranial aneurysm. J Endovasc Ther 2004;11:411–3.
Crossref| PubMed - Ruzsa Z, Nemes B, Pintér L, et al. A randomised comparison of transradial and transfemoral approach for carotid artery stenting: RADCAR (RADial access for CARotid artery stenting) study. EuroIntervention 2014;10:381–91.
Crossref| PubMed - Peitz GW, Kura B, Johnson JN, Grandhi R. Transradial approach for deployment of a flow diverter for an intracranial aneurysm in a patient with a type-3 aortic arch. J Vasc Interv Neurol 2017;9:42–4.
PubMed - Chen SH, Snelling BM, Shah SS, et al. Transradial approach for flow diversion treatment of cerebral aneurysms: a multicenter study. J Neurointerv Surg 2019;11:796–800.
Crossref| PubMed - Daou B, Chalouhi N, Tjoumakaris S, et al. Alternative access for endovascular treatment of cerebrovascular diseases. Clin Neurol Neurosurg 2016;145:89–95.
Crossref| PubMed - Pinter L, Cagiannos C, Ruzsa Z, et al. Report on initial experience with transradial access for carotid artery stenting. J Vasc Surg 2007;45:1136–41.
Crossref| PubMed - Wasilewski J, Głowacki J, Polonski L. Not at random location of atherosclerotic lesions in thoracic aorta and their prognostic significance in relation to the risk of cardiovascular events. Pol J Radiol 2013;78:38–42.
Crossref| PubMed - Gu X, He Y, Li Z, et al. Relation between the incidence, location, and extent of thoracic aortic atherosclerosis detected by transesophageal echocardiography and the extent of coronary artery disease by angiography. Am J Cardiol 2011;107:175–8.
Crossref| PubMed - Mitchell MD, Hong JA, Lee BY, et al. Systematic review and cost-benefit analysis of radial artery access for coronary angiography and intervention. Circ Cardiovasc Qual Outcomes 2012;5:454–62.
Crossref| PubMed - Jolly SS, Yusuf S, Cairns J, et al. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet 2011;377:1409–20.
Crossref| PubMed - Singh S, Singh M, Grewal N, Khosla S. Transradial vs transfemoral percutaneous coronary intervention in ST-segment elevation myocardial infarction: a systemic review and meta-analysis. Can J Cardiol 2016;32:777–90.
Crossref| PubMed - Agostoni P, Biondi-Zoccai GGL, De Benedictis ML, et al. Radial versus femoral approach for percutaneous coronary diagnostic and interventional procedures: Systematic overview and meta-analysis of randomized trials. J Am Coll Cardiol 2004;44:349–56.
Crossref| PubMed - Snelling BM, Sur S, Shah SS, et al. Transradial cerebral angiography: techniques and outcomes. J Neurointerv Surg 2018;10:874–81.
Crossref| PubMed - Zussman BM, Tonetti DA, Stone J, et al. Maturing institutional experience with the transradial approach for diagnostic cerebral arteriography: overcoming the learning curve. J Neurointerv Surg 2019;11:1235–8.
Crossref| PubMed - Joshi KC, Beer-Furlan A, Crowley RW, et al. Transradial approach for neurointerventions: A systematic review of the literature. J Neurointerv Surg 2020;12:886–92.
Crossref| PubMed - Koutouzis MJ, Maniotis CD, Avdikos G, et al. ULnar Artery Transient Compression Facilitating Radial Artery Patent Hemostasis (ULTRA): a novel technique to reduce radial artery occlusion after transradial coronary catheterization. J Invasive Cardiol 2016;28:451–4.
PubMed - Caputo RP, Tremmel JA, Rao S, et al. Transradial arterial access for coronary and peripheral procedures: executive summary by the transradial committee of the SCAI. Catheter Cardiovasc Interv 2011;78:823–39.
Crossref| PubMed - Stella PR, Kiemeneij F, Laarman GJ, et al. Incidence and outcome of radial artery occlusion following transradial artery coronary angioplasty. Cathet Cardiovasc Diagn 1997;40:156–8.
Crossref| PubMed - van der Heijden DJ, van Leeuwen MAH, Ritt MJPF, et al. Hand sensibility after transradial arterial access: an observational study in patients with and without radial artery occlusion. J Vasc Interv Radiol 2019;30:1832–9.
Crossref| PubMed - Sciahbasi A, Rigattieri S, Sarandrea A, et al. Radial artery occlusion and hand strength after percutaneous coronary procedures: results of the HANGAR study. Catheter Cardiovasc Interv 2016;87:868–74.
Crossref| PubMed - Tizón-Marcos H, Barbeau GR. Incidence of compartment syndrome of the arm in a large series of transradial approach for coronary procedures. J Interv Cardiol 2008;21:380–4.
Crossref| PubMed - Jaroenngarmsamer T, Bhatia KD, Kortman H, et al. Procedural success with radial access for carotid artery stenting: Systematic review and meta-analysis. J Neurointerv Surg 2020;12:87–93.
Crossref| PubMed - Polguj M, Stefanczyk L, Topol M. The epidemiological, morphological, and clinical aspects of the aberrant right subclavian artery (arteria lusoria). In: Kasenga F, ed. Epidemiology of Communicable and Non-Communicable Diseases – Attributes of Lifestyle and Nature on Humankind. London: IntechOpen, 2016.
Crossref - Valgimigli M, Campo G, Penzo C, et al. Transradial coronary catheterization and intervention across the whole spectrum of Allen test results. J Am Coll Cardiol 2014;63:1833–41.
Crossref| PubMed - Valgimigli M, Frigoli E, Leonardi S, et al. Radial versus femoral access and bivalirudin versus unfractionated heparin in invasively managed patients with acute coronary syndrome (MATRIX): final 1-year results of a multicentre, randomised controlled trial. Lancet 2018;392:835–48.
Crossref| PubMed - Uhlemann M, Möbius-Winkler S, Mende M, et al. The Leipzig prospective vascular ultrasound registry in radial artery catheterization: impact of sheath size on vascular complications. JACC Cardiovasc Interv 2012;5:36–43.
Crossref| PubMed - Beyer AT, Ng R, Singh A, et al. Topical nitroglycerin and lidocaine to dilate the radial artery prior to transradial cardiac catheterization: a randomized, placebo-controlled, double-blind clinical trial: the PRE-DILATE Study. Int J Cardiol 2013;68:2575–8.
Crossref| PubMed - Candemir B, Kumbasar D, Turhan S, et al. Facilitation of radial artery cannulation by periradial subcutaneous administration of nitroglycerin. J Vasc Interv Radiol 2009;20:1151–6.
Crossref| PubMed - Ezhumalai B, Satheesh S, Jayaraman B. Effects of subcutaneously infiltrated nitroglycerin on diameter, palpability, ease-of-puncture and pre-cannulation spasm of radial artery during transradial coronary angiography. Indian Heart J 2014;66:593–7.
Crossref| PubMed - Horie K, Tada N, Isawa T, et al. A randomised comparison of incidence of radial artery occlusion and symptomatic radial artery spasm associated with elective transradial coronary intervention using 6.5 Fr SheathLess Eaucath Guiding Catheter vs. 6.0 Fr Glidesheath Slender. EuroIntervention 2018;20:2018–25.
Crossref| PubMed - Dahal K, Sharma S, Yousuf A, et al. A comparison of standard versus low dose heparin on access-related complications after coronary angiography through radial access: a meta-analysis of randomized controlled trials. Cardiovasc Revasc Med 2018;19:575–9.
Crossref| PubMed - Hahalis GN, Leopoulou M, Tsigkas G, et al. Multicenter randomized evaluation of high versus standard heparin dose on incident radial arterial occlusion after transradial coronary angiography: the SPIRIT OF ARTEMIS Study. JACC Cardiovasc Interv 2018;11:2241–50.
Crossref| PubMed - Pancholy SB. Comparison of the effect of intra-arterial versus intravenous heparin on radial artery occlusion after transradial catheterization. Am J Cardiol 2009;104:1083–5.
Crossref| PubMed - Rosencher J, Chaïb A, Barbou F, et al. How to limit radial artery spasm during percutaneous coronary interventions: The spasmolytic agents to avoid spasm during transradial percutaneous coronary interventions (SPASM3) study. Catheter Cardiovasc Interv 2014;84:766–71.
Crossref| PubMed - Kiemeneij F, Vajifdar BU, Eccleshall SC, et al. Evaluation of a spasmolytic cocktail to prevent radial artery spasm during coronary procedures. Catheter Cardiovasc Interv 2003;58:281–4.
Crossref| PubMed - Chen CW, Lin CL, Lin TK, Lin CD. A simple and effective regimen for prevention of radial artery spasm during coronary catheterization. Cardiology 2006;105:43–7.
Crossref| PubMed - Kwok CS, Rashid M, Fr aser D, et al. Intra-arterial vasodilators to prevent radial artery spasm: a systematic review and pooled analysis of clinical studies. Cardiovasc Revasc Med 2015;16:484–90.
Crossref| PubMed - Dangoisse V, Guédès A, Chenu P, et al. Usefulness of a gentle and short hemostasis using the transradial band device after transradial access for percutaneous coronary angiography and interventions to reduce the radial artery occlusion rate (from the Prospective and Randomized CRASOC I, II, and III Studies). Am J Cardiol 2017;120:374–9.
Crossref| PubMed - Pancholy SB, Bernat I, Bertrand OF, Patel TM. Prevention of radial artery occlusion after transradial catheterization. JACC Cardiovasc Interv 2016;9:1992–9.
Crossref| PubMed - Bernat I, Bertrand OF, Rokyta R, et al. Efficacy and safety of transient ulnar artery compression to recanalize acute radial artery occlusion after transradial catheterization. Am J Cardiol 2011;107:1698–701.
Crossref| PubMed - Kiemeneij F. Left distal transradial access in the anatomical snuffbox for coronary angiography (ldTRA) and interventions (ldTRI). EuroIntervention 2017;13:851–7.
Crossref| PubMed - Brunet M-C, Chen SH, Sur S, et al. Distal transradial access in the anatomical snuffbox for diagnostic cerebral angiography. J NeuroIntervent Surg 2019;11:710–3.
Crossref| PubMed - Patel P, Majmundar N, Bach I, et al. Distal transradial access in the anatomic snuffbox for diagnostic cerebral angiography. Am J Neuroradiol 2019;40:1526–8.
Crossref| PubMed - Koutouzis M, Kontopodis E, Tassopoulos A, et al. Distal versus traditional radial approach for coronary angiography. Cardiovasc Revas Med 2019;20:678–80.
Crossref| PubMed - Kawamura Y, Yoshimachi F, Nakamura N, et al. Impact of dedicated hemostasis device for distal radial arterial access with an adequate hemostasis protocol on radial arterial observation by ultrasound. Cardiovasc Interv Ther 2020; epub ahead of press.
Crossref| PubMed - Deftereos S, Giannopoulos G, Raisakis K, et al. Moderate procedural sedation and opioid analgesia during transradial coronary interventions to prevent spasm: A prospective randomized study. JACC Cardiovasc Interv 2013;6:267–73.
Crossref| PubMed - Ho HH, Jafary FH, Ong PJ. Radial artery spasm during transradial cardiac catheterization and percutaneous coronary intervention: Incidence, predisposing factors, prevention, and management. Cardiovasc Revasc Med 2012;13:193–5.
Crossref| PubMed - Roa JA, Ortega-Gutierrez S, Martinez-Galdamez M, et al. Transcirculation approach for endovascular embolization of intracranial aneurysms, arteriovenous malformations, and dural fistulas: a multicenter study. World Neurosurg 2020;134:e1015–27.
Crossref| PubMed - Chapot R, Stracke P, Velasco A, et al. The pressure cooker technique for the treatment of brain AVMs. J Neuroradiol 2014;41:87–91.
Crossref| PubMed - Chen SH, Snelling BM, Sur S, et al. Transradial versus transfemoral access for anterior circulation mechanical thrombectomy: comparison of technical and clinical outcomes. J Neurointerv Surg 2019;11:874–8.
Crossref| PubMed - Hill MD, Brooks W, Mackey A, et al. Stroke after carotid stenting and endarterectomy in the Carotid Revascularization Endarterectomy versus Stenting Trial (CREST). Circulation 2012;126:3054−61.
Crossref| PubMed - Macdonald S, Lee R, Williams R, Stansby G. Towards safer carotid artery stenting a scoring system for anatomic suitability. Stroke 2009;40:1698–703.
Crossref| PubMed - Meissner I, Whisnant JP, Khandheria BK, et al. Prevalence of potential risk factors for stroke assessed by transesophageal echocardiography and carotid ultrasonography: the SPARC study. Mayo Clin Proc 1999;74:862–9.
Crossref| PubMed - Hammer FD, Lacroix V, Duprez T, et al. Cerebral microembolization after protected carotid artery stenting in surgical high-risk patients: results of a 2-year prospective study. J Vasc Surg 2005;42:847–53.
Crossref| PubMed - Etxegoien N, Rhyne D, Kedev S, et al. The transradial approach for carotid artery stenting. Catheter Cardiovasc Interv 2012;80:1081–7.
Crossref| PubMed - Kedev S. Transradial carotid artery stenting: examining the alternatives when femoral access is unavailable. Interv Cardiol 2014;6:463–75.
