Mustafa Abbas, Trevor Cleveland
|
Abstract Carotid interventions, carotid endarterectomy and carotid artery stenting (CAS) have proven to be effective treatments for the prevention of ischaemic stroke in recently symptomatic patients. Most studies were conducted before the development of strict statin guidance and the systematic use of antiplatelet drugs. The advances in medical treatments have raised questions regarding the benefit of carotid endarterectomy or CAS, especially for high-grade asymptomatic internal carotid artery stenosis. Reviewing the literature indicates that carotid artery stenosis of any degree is a relatively weak predictor of ipsilateral stroke, in the absence of recent symptoms referable to the carotid disease. This risk does not appear reduced by revascularisation by CAS if added to modern day best medical therapy. On-going trials are key to understanding if current techniques can provide an additional benefit Keywords: Carotid artery stenting, carotid endarterectomy, carotid artery stenosis, best medical therapy, asymptomatic carotid artery stenosis, internal carotid artery, coronary artery bypass grafting, transient ischaemic attack, MRI, embolic protection devices. Disclosure: The authors have no conflicts of interest to declare. Received: 02 August Accepted: 18 April 2022 Published online: 22 June 2022 Correspondence Details: :Mustafa Abbas, Sheffield Vascular Institute, Northern General Hospital, Herries Rd, Sheffield S5 7AU, UK. E: mustafaabbas1@doctors.org.uk Copyright Statement:
This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly
|
Carotid artery stenosis (CS) is usually classified as being mild (<50% left main stem [LMS] loss), moderate (50–69% LMS loss) or severe (≥70% LMS loss).5 While prevalence is relatively low in the general population, it increases with age, affecting up to 12.5% of men and 6.9% of women >70 years of age.6
Although certain ethnic subgroups are at highest risk, notably white and Native American people, the prevalence of significant asymptomatic carotid artery stenosis (ACS) is found to be increased in patients with peripheral artery disease (up to 39% of this group).7 People who have undergone coronary artery bypass grafting (CABG) have a 26.4% prevalence of moderate ACS and 8.6% have severe ACS.5 Individuals who have ACS experience a 3% yearly risk of having a stroke, which equates to a >50% increased relative risk compared with the general population.2
CS is most frequently the result of narrowing of the carotid artery LMS caused by atherosclerotic plaque formation within the artery wall. This may result in symptoms such as ipsilateral carotid territory ischaemic stroke, transient ischaemic attack (TIA) or amaurosis fugax. ACS is considered in individuals who have CS without a history of the localising events or lack of the symptoms for at least 6 months.8 Modern non-invasive imaging modalities – most notably duplex ultrasound – are performed for a variety of reasons, which has resulted in asymptomatic stenosis being more frequently detected. This leads to the question of whether these people should be considered for treatment of their asymptomatic carotid stenosis by stenting, in addition to medical therapy.
Furthermore, there are some situations where stroke risk is considered to be high, in the presence of an ACS, such as when undergoing another intervention. The relationship between stroke and CABG is the area that has attracted the most attention.
Therapeutic options to treat the carotid stenosis include carotid endarterectomy (CEA) and medical therapy, carotid artery stenting (CAS) and medical therapy or medical therapy alone. There is strong evidence that CAS or CEA for symptomatic ICA stenosis of >70%, in addition to best medical therapy (BMT), is beneficial. The maximum benefit of intervention after an ipsilateral TIA or non-disabling stroke accrues if delivered in the first 2 weeks after an event.9 Nevertheless, the long-term prevention of stroke in asymptomatic patients remains ambiguous regarding whether either of CEA or CAS, in addition to BMT, is better than BMT alone. The available data to inform balancing the risks and benefits of the use of CAS in ACS, are considered below. Some of those data from trials not directly designed for the investigation of ACS and CAS, with many trials including both symptomatic and asymptomatic patients and both CEA and CAS.
Published Randomised Trials
The ACAS and ACST 1 trials were published in 1995 and 2004, respectively, and have remained the basis for most of the subsequent guidelines.10,11 However, the outcomes after BMT, CAS and CEA have significantly improved in the last 20 years and the data from these two trials, which have supported every practice guideline since 1995, may no longer be appropriate for use in 2022.4
In the ACAS trial, people with ACS ≥60% were investigated. Patients were randomised to receive either medical therapy in addition to CEA, or medical therapy with 325 mg aspirin daily along with coronary vascular disease risk modification. Based on a mean follow up of 2.7 years, it was estimated that the 5-year risk of stroke or death was 5.1% in the CEA with medical therapy group and 11% in the medical therapy alone group.5 On that basis, CEA in addition to medical therapy is often recommended, provided that the risk of the CEA is kept low. For CAS to be considered as an alternative to CEA, it needs to be delivered with at least similar rates of stroke. This has been shown to be the case in the ACST-2 trial where the complications of CAS and CEA are similar.12
In the ACST 1 trial, participants with asymptomatic CS ≥60% were randomised to BMT with or without concomitant CEA. After excluding perioperative strokes, CEA was associated with a 6.4% rate of carotid territory stroke compared to 11.8% in the BMT group at 5 years. The risk of perioperative stroke in ACST 1 was 2.8% (1.5% in ACAS) and – again – CAS needs to be similar or better to offer benefit.11
The CREST 1 study recruited 2,502 patients and used a primary endpoint of periprocedural stroke, MI, death or long-term rates of ipsilateral stroke. There was no difference in the estimated 4-year rates of the primary endpoint in CEA and carotid stenting treatment (in addition to medical therapy) for symptomatic and asymptomatic patients.13 During the periprocedural period, there was a higher risk of MI with CEA against a higher risk of all stroke with CAS. Also noted in this study was the effect of age on outcomes, whereby patients aged <70 years appeared to have lower complication rates with CAS while patients aged >70 years appeared to have higher complication rates with CAS compared to CEA.14 The authors found that the annual risks of stroke in asymptomatic patients including the periprocedural risk were 1.2% with CAS and 0.95% with CEA.7
This trial did not have a comparator group of medical therapy alone for patients with ACS. Similar results were shown in ACT-I.15 In this trial (primary endpoint of stroke, MI and death within 30 days of the procedure or ipsilateral stroke within a year and follow up to 5 years), there was no significant difference in asymptomatic patients after CEA or stenting and the procedural risk threshold was below 3%. This trial also did not have a medical therapy arm. However, it does support the concept that CEA and CAS offer a similar utility in stroke prevention following successful treatment.
The SPACE-2 trial aimed to compare BMT plus intervention (CAS or CEA) in patients with asymptomatic carotid stenosis with BMT alone. Unfortunately, the study was stopped prematurely after randomising 513 patients because of recruitment difficulties. One important reason why SPACE-2 had poor recruitment was the unwillingness of patients to accept BMT alone (they had already been prepared to undergo an intervention using either CAS or CEA), particularly when these patients would have received BMT in all three study arms anyway.
However, at termination of the trial, there was no superiority of CEA or CAS against the BMT in the primary prevention of ischaemic stroke in patients with an asymptomatic carotid stenosis up to 1 year after treatment. As a result of the early termination of the trial, the sample size was too small for statistical confidence. It did indicate that there were no apparent differences the between the CAS or CEA in terms of safety during the first year after treatment. A 5-year follow-up is on-going.6
The findings of ACST-2, which compared CEA with CAS for long-term stroke prevention in patients with severe ACS on BMT, has recently been published. This study showed no significant difference in the risk of adverse, procedure-related events for CAS and CEA. For both treatments, 1% of patients had a disabling stroke or died within 30 days (15 in the CAS group and 18 in the CEA group) and 2% had a non-disabling stroke (48 in the CAS group and 29 in the CEA group). In addition, the number of strokes that occurred in the participants over the 5-year follow-up period was similar for CAS and CEA. Non-procedural fatal or disabling stroke occurred in 2.5% of patients in each group, and the rate of strokes was 5.3% in the CAS group, and 4.5% in the CEA group. Other complications including MI (fatal and non-fatal) were approximately similar (0.3% with CAS and 0.7% with CEA). BMT can also reduce stroke rates but – even when receiving it – patients with severe CAS might have a risk of approximately 1% per year of disabling stroke or death. Hence, in addition to BMT, carotid procedures are still considered appropriate for many patients.12 Summarised data from the studies described above are in Table 1.
Review of Meta-analysis Data
A review of more recent meta-analyses shows that the short-term vascular endpoints at 30 days (i.e. stroke, MI or vascular death) are lower with BMT compared with any surgical or endovascular intervention. This is the result of the peri-operative risk associated with either intervention.
The long-term risk of stroke up to 10 years is lower with CEA than BMT, while there is no difference in the risk of death between the interventional treatment (CAS and CEA) and BMT.16,17 No studies are available to compare CAS with BMT alone, but long-term outcomes are considered to be probably no different between CEA and CAS.16 A systematic review and meta-analysis that included data from 56 studies on the evidence on treating and screening ACS, was conducted by the US Preventive Services Task Force.18 The authors compared carotid revascularisation (CEA and CAS) with medical therapy. The data analysis showed an absolute risk reduction of 5.5% for any non-perioperative stroke over approximately 5 years follow-up. However there was a 2.4% risk of perioperative (30 days) stroke or death after CEA and CAS. This study concluded that there is no overall benefit of CEA or CAS in ACS.18
The Role of Best Medical Therapy in Asymptomatic Carotid Artery Stenosis
Regardless of treatment type (CEA or CAS), the consensus of current guidelines is that intervening in ACS is indicated when the life expectancy of the patients is >5 years and the perioperative risk is <3% (class IIa; level of evidence A) and that CAS might be considered in highly selected patients (class IIb; level of evidence B).19 However, the question arises whether modern BMT confers as much benefit in ACS as does CAS, and if so, whether the peri-procedural risk is worth taking.
BMT includes lifestyle modifications promoting a healthy lifestyle, including smoking cessation, physical activity, a healthy diet and well-controlled blood pressure. These are the cornerstones for primary and secondary atherosclerotic prevention, including that of stroke. According to the European Society of Cardiology, smoking increases the risk of heart disease and stroke fivefold in people <50 years and doubles the risk in those >60 years. The lifestyle guidelines from the American Heart Association in 2013 advocated, alongside smoking cessation, a dietary pattern that encourages the intake of fruits, vegetables and whole grains with a decreased intake of sweets, red meat and saturated fat. Guidelines also encourage increased use of the Mediterranean diet, supplemented by nuts (walnuts, hazelnuts and almonds) or by extra virgin olive oil in order to reduce the stroke risk, along with increasing the intake of B vitamins to lower homocysteine levels.20
Summary of Trial Characteristics
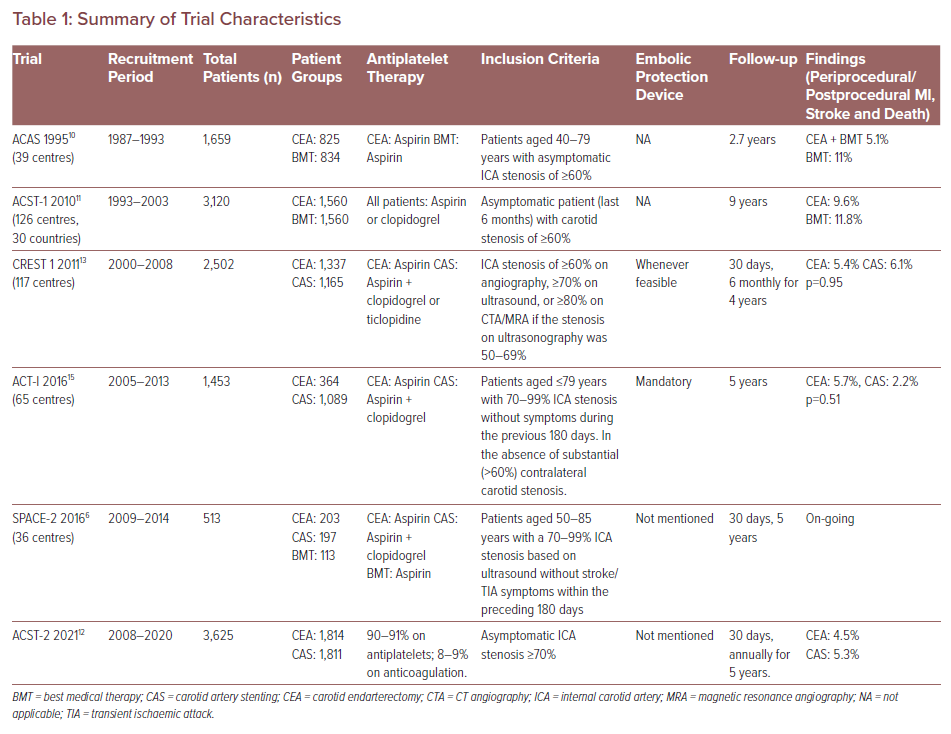
Somewhat surprisingly, data supporting the use of antiplatelet therapy for primary stroke prevention in ACS are limited. Based on limited studies, such as the Women’s Health Study and the Asymptomatic Cervical Bruit Study, the current guidelines include a class I recommendation for aspirin therapy in patients with ACS, but there are no data supporting dual antiplatelet therapy for ACS in the absence of other cardiovascular diseases.7
There is growing evidence that only a minority of patients receiving BMT for asymptomatic ICA stenosis will benefit from intervention given the significant improvement in the preventative management of cardiovascular diseases, particularly with the development and use of antiplatelets and lipid-lowering therapy, alongside improvements in lifestyle care. This is demonstrated by the ACAS trial, in which the 5-year risk for ipsilateral stroke in 1995 was 11% in those receiving BMT. This was halved to 5.3% by 2004 and to 3.6% by 2010 as shown in the ACST 1 trial. This corresponds to an overall relative risk reduction of approximately 70% over 15 years.
Patients with ACS also have a substantial frequency of associated coronary artery atherosclerosis: MI is about half as frequent as stroke, while the risk of cardiovascular death exceeds that of stroke. Aggressive medical management of ACS offers the additional benefit of prevention of coronary events.17
Spence et al. argued that the annual risk of ipsilateral stroke with modern BMT should be as low as 0.5% per year in ACS; based on trials, such as ACT 1 and CREST 1, outcomes for patients who have interventions trail behind BMT after taking peri-procedural risks into account.21,22 The outcomes of intervention are now comparable to medical therapy in terms of long-term stroke prevention. However, the periprocedural risks still exceed the risks of medical therapy alone.
Therefore, only ACS patients with a factor that renders them at higher risk of stroke would be likely to benefit from interventions such as CAS or CEA. It has been proposed that there may be subgroups who may benefit, which may be based on evidence of embolic activity using trans-cranial Doppler, MRI plaque morphology or plaque inflammation on PET CT.21
The recent systematic review and meta-analysis by Howard et al. indicates that the more severe degrees of ACS may benefit from intervention, but not those considered to have moderate ACS.23 The potential of benefit in these groups, who are considered to be at higher risk of stroke, has yet to be thoroughly investigated or the case proven.
Future Randomised Controlled Trials
There are a number of on-going trials that are attempting to answer some of the uncertainties in relation to the benefit (or not) of CAS or CEA in addition to BMT for ACS. The results of these trials are pivotal in understanding patient benefits and risks.
The CREST-2 trial (NCT02089217) has been designed to allow and facilitate decision making regarding the optimal management of high-grade ACS. It is comparing stroke prevention by BMT alone against revascularisation (CEA or CAS) with BMT in patients with a 70–99% ACS. The results of this trial will be available after recruitment completion, which was expected to be in December 2020. Follow-up will be required, and, as with many trials currently, there may be delays because of the COVID-19 pandemic.
In ECST-2 (NCT00883402), where CEA and CAS are being compared with BMT in patients with severe ACS. ACTRIS (NCT02841098) will compare CEA with BMT against BMT alone. CAS is not included in the trial, therefore further direct information of CAS in asymptomatic patients will not be available from this study.
When we have the benefit of the results of these trials it is hoped that there will be more clarity regarding management of patients with ACS – particularly with the use of new-designed stents – potentially increasing the safety and effectiveness of the CAS.
Of interest there is a new study, CREST-H, which is designed to investigate the decline of cognition due to a reduction in the cerebral blood flow secondary to high-grade carotid stenosis, which is otherwise ‘asymptomatic’ in terms of TIA or stroke. The trialists explore whether revascularisation of a haemodynamically significant carotid stenosis can alter the course of cognitive decline.24
Patients Undergoing CABG with Concomitant Asymptomatic Carotid Disease
Patients who fall into this group are considered to have a high risk of stroke at the time of their coronary artery surgery. There has been much debate in the literature as to the potential benefit that may be gained by having the carotid disease treated either before, or at the same time as, the coronary artery bypass. One of the major problems with offering CEA prior to CABG is that there is a higher risk of myocardial events at the time of CEA. However, if the CEA is performed simultaneously with the CABG, the stroke risk may remain high.25
Analysis of data including 2,813 patients from a prospective multicentre observational study showed that in patients with ACS who are undergoing isolated CABG, the risk of postoperative stroke is significant only when the stenosis is ≥90%, with an incidence of stroke in these patients of approximately 7.0%.26 As there is potential for differing levels of carotid disease on either the left or right side, the total burden of carotid artery disease was addressed by Naylor et al.27 This analysis of the data concluded that there was an increase in the CABG-related stroke rate as the total burden of carotid disease increased.
Because of the perceived reduced risk of cardiac events occurring when CAS is performed prior to CABG rather than CEA (which is supported by trials such as CREST), CAS has been proposed as a preferable method for treating high-grade carotid disease prior to CABG. This was reviewed in 2017 by Paraskevas et al. who included 31 studies of 2,727 patients in whom 80% were neurologically asymptomatic with unilateral stenoses.28 This meta-analysis suggested that overall 30-day outcomes after CAS and CABG or after CEA and CABG are broadly similar. However, in patients with a history of TIA/stroke, staged or same-day CEA and CABG is considered to be the preferred option over CAS and CABG. For the majority of the patients who are asymptomatic, the risks following CABG and CAS are 7.9% for death/stroke and an 8.8% risk of death/stroke/MI. This exceeds the risk of death/stroke in patients who are undergoing isolated CABG (no prophylactic CAS/CEA), where, in the presence of a >50% carotid stenosis, the prevalence of stroke within 30 days of CABG was 7.4%, while death/stroke was 8.3%. The conclusion was that prophylactic CAS in asymptomatic patients does not add any additional benefits over isolated CABG in this group of patients. However, from the work of Naylor et al., it may still be the case that those with a higher burden of disease do indeed obtain benefit if their ACS is treated prior to CABG. However, Santarpino et al. argue the opposite, that asymptomatic, severe CS has a low prevalence and when left untreated is associated with a relatively low risk of stroke. This may argue that preoperative screening for ACS before CABG may not be justified.26
Conclusion
Carotid interventions have proven to be an effective treatment in preventing ischaemic stroke in symptomatic patients. However, the majority of studies for asymptomatic patients – and indeed recently-symptomatic patients – were conducted before the advances in modern medical treatment with lipid-lowering therapy, antiplatelet treatment, antihypertensives and good diabetic control, together with lifestyle modifications such as smoking cessation, regular exercise and a well-balanced diet. This change in BMT is likely responsible for the 33% relative risk reduction in the 5-year risk of any stroke noted in ACST (published in 2004) compared to the earlier ACAS trial (published in 1995), which has raised questions regarding the benefit of CEA or CAS in the context of asymptomatic ICA stenosis.29
This review of the literature indicates that CS of any degree is a relatively weak predictor of ipsilateral stroke, in the absence of recent symptoms referable to the carotid disease, and that this risk is not reduced by revascularisation if added to best medical therapy. It is possible that there are some subgroups who are at higher risk of stroke and the evidence in support of CAS prior to CABG, even in high level disease, remains controversial. Evidence from further randomised controlled trials will be key.
There are on-going trials currently recruiting patients. CREST-2 will test CEA and CAS for asymptomatic ICA stenosis versus BMT. In ECST-2, CEA and CAS are being compared with BMT in patients with severe ACS and ACTRIS compares CEA with BMT against BMT alone. The results of these trials are yet to be published, at which time they will hopefully further clarify the role of carotid interventions.
Current data do not support the routine treatment of asymptomatic carotid stenosis with CAS, outside the realms of research, and in the small group of patients at particularly high risk from their stenosis, such as patients undergoing CABG with bilateral high-grade stenosis or those with severe stenosis.
References
-
-
- Janczak D, Malinowski M, Ziomek A, et al. Carotid artery stenting versus endarterectomy for the treatment of both symptomatic and asymptomatic patients with carotid artery stenosis: 2 years’ experience in a high-volume center. Adv Clin Exp Med 2018;27:1691–5.
Crossref| PubMed - Gaba K, Ringleb PA, Halliday A. Asymptomatic carotid stenosis: intervention or best medical therapy? Curr Neurol Neurosci Rep 2018;18:80.
Crossref| PubMed - Naylor AR. Why is the management of asymptomatic carotid disease so controversial? Surgeon 2015;13:34–43.
Crossref| PubMed - Lal BK, Meschia JF, Brott TG. Clinical need, design, and goals for the Carotid Revascularization and Medical Management for Asymptomatic Carotid Stenosis trial. Semin Vasc Surg 2017;30:2–7.
Crossref| PubMed - Dharmadhikari S, Chaturvedi S. Medical and revascularization therapies for asymptomatic carotid stenosis. Curr Atheroscler Rep 2015;17:44.
Crossref| PubMed - Reiff T, Eckstein HH, Mansmann U, et al. Angioplasty in asymptomatic carotid artery stenosis vs. endarterectomy compared to best medical treatment: one-year interim results of SPACE-2. Int J Stroke 2020;15:638–49.
Crossref| PubMed - Aday AW, Beckman JA. Medical management of asymptomatic carotid artery stenosis. Prog Cardiovasc Dis 2017;59:585–90.
Crossref| PubMed - Moresoli P, Habib B, Reynier P, et al. Carotid stenting Versus endarterectomy for asymptomatic carotid artery stenosis: a systematic review and meta-analysis. Stroke 2017;48:2150–7.
Crossref| PubMed - Rothwell PM, Eliasziw M, Gutnikov SA, et al. Endarterectomy for symptomatic carotid stenosis in relation to clinical subgroups and timing of surgery. Lancet 2004;363:915–24.
Crossref| PubMed - Walker MD, Marler JR, Goldstein M, et al. Endarterectomy for asymptomatic carotid artery stenosis. Executive Committee for the Asymptomatic Carotid Atherosclerosis Study. JAMA 1995;273:1421–8.
Crossref| PubMed - Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet 2004;363:1491–502.
Crossref| PubMed - Halliday A, Bulbulia R, Bonati LH, et al. Second Asymptomatic Carotid Surgery Trial (ACST-2): a randomised comparison of carotid artery stenting versus carotid endarterectomy. Lancet 2021;398:1065–73.
Crossref| PubMed - Brott TG, Hobson RW, Howard G, et al. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med 2010;363:11–23.
Crossref| PubMed - Brott TG, Howard G, Roubin GS, et al. Long-term results of stenting versus endarterectomy for carotid-artery stenosis. N Engl J Med 2016;374:1021–31.
Crossref| PubMed - Rosenfield K, Matsumura JS, Chaturvedi S, et al. Randomized trial of stent versus surgery for asymptomatic carotid stenosis. N Engl J Med 2016;374:1011–20.
Crossref| PubMed - Barkat M, Roy I, Antoniou SA, et al. Systematic review and network meta-analysis of treatment strategies for asymptomatic carotid disease. Sci Rep 2018;8:4458.
Crossref| PubMed - Galyfos G, Sachsamanis G, Anastasiadou C, et al. Carotid endarterectomy versus carotid stenting or best medical treatment in asymptomatic patients with significant carotid stenosis: a meta-analysis. Cardiovasc Revasc Med 2019;20:413–23.
Crossref| PubMed - Gokaldas R, Singh M, Lal S, et al. Carotid stenosis: from diagnosis to management, where do we stand? Curr Atheroscler Rep 2015;17:480.
Crossref| PubMed - Hart RG, Ng KH. Stroke prevention in asymptomatic carotid artery disease: revascularization of carotid stenosis is not the solution. Pol Arch Med Wewn 2015;125:363–9.
Crossref| PubMed - Eckel RH, Jakicic JM, Ard JD, et al. 2013 AHA/ACC guideline on lifestyle management to reduce cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014;129 (25 Suppl 2):S76–99.
Crossref| PubMed - Spence JD, Song H, Cheng G. Appropriate management of asymptomatic carotid stenosis. Stroke Vasc Neurol 2016;1:64–71.
Crossref| PubMed - Spence JD. Management of patients with an asymptomatic carotid stenosis-medical management, endovascular treatment, or carotid endarterectomy? Curr Neurol Neurosci Rep 2016;16:3.
Crossref| PubMed - Howard DPJ, Gaziano L, Rothwell PM, Oxford Vascular Study. Risk of stroke in relation to degree of asymptomatic carotid stenosis: a population-based cohort study, systematic review, and meta-analysis. Lancet Neurol 2021;20:193–202.
Crossref| PubMed - Marshall RS, Lazar RM, Liebeskind DS, et al. Carotid revascularization and medical management for asymptomatic carotid stenosis – hemodynamics (CREST-H): study design and rationale. Int J Stroke 2018;13:985–91.
Crossref| PubMed - Garg A, Bansal AR, Singh D, et al. Combining carotid endarterectomy with off-pump coronary artery bypass graft surgery is safe and effective. Ann Indian Acad Neurol 2015;18:419–23.
Crossref| PubMed - Santarpino G, Nicolini F, De Feo M, et al. Prognostic impact of asymptomatic carotid artery stenosis in patients undergoing coronary artery bypass grafting. Eur J Vasc Endovasc Surg 2018;56:741–8.
Crossref| PubMed - Naylor AR, Mehta Z, Rothwell PM, Bell PRF. Carotid artery disease and stroke during coronary artery bypass: a critical review of the literature. Eur J Vasc Endovasc Surg 2002;23:283–94.
Crossref| PubMed - Paraskevas KI, Nduwayo S, Saratzis AN, Naylor AR. Carotid stenting prior to coronary bypass surgery: an updated systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2017;53:309–19.
Crossref| PubMed - Naylor AR, Gaines PA, Rothwell PM. Who benefits most from intervention for asymptomatic carotid stenosis: patients or professionals? Eur J Vasc Endovasc Surg 2009;37:625–32.
Crossref| PubMed
- Janczak D, Malinowski M, Ziomek A, et al. Carotid artery stenting versus endarterectomy for the treatment of both symptomatic and asymptomatic patients with carotid artery stenosis: 2 years’ experience in a high-volume center. Adv Clin Exp Med 2018;27:1691–5.
-
