Raleene Gatmaitan, Keagan Werner-Gibbings, Tommaso Donati, Prakash Saha, Stephen Black
|
Abstract May–Thurner syndrome (MTS) is caused by compression of the left iliac vein by the right iliac artery, leading to clinical manifestations of outflow obstruction in the lower limb and deep vein thrombosis. There have been increasing reports of iatrogenic MTS caused by medical implants. The authors report the case of a 60-year-old man who developed MTS after stenting of the right common iliac artery. Due to the debilitating nature of the patient’s symptoms of venous congestion in the left leg, he proceeded with endovascular venoplasty and venous stent insertion with concurrent intra-arterial balloon angioplasty of the existing right common iliac artery stent. Technical success and primary patency of arterial and venous stents were achieved. The patient remained asymptomatic at 6 weeks and 3 months follow-up and arterial and venous stents were found to be patent on duplex ultrasound. Surgical management of MTS may include thrombolysis, thrombectomy, venoplasty and stenting of the left common iliac vein. Care must be taken to preserve existing medical implants during treatment of MTS. The authors demonstrate that concurrent angioplasty of the right common iliac artery during treatment of the vein is an effective method of preventing arterial stent disruption during surgical management of MTS. Keywords: May–Thurner syndrome, venoplasty, stent, deep vein thrombosis, iatrogenic Disclosure: PS is on the editorial board and SB is editor-in-chief of Vascular & Endovascular Review; this did not influence peer review. All other authors have no conflicts of interest to declare. Received: Accepted: Published online: Correspondence Details: Keagan Werner-Gibbings, Nepean Hospital, Derby St, Kingswood, NSW 2747, Australia. E: keagan.werner-gibbings@health.nsw.gov.au Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly. |
May–Thurner syndrome (MTS) is a well-known condition where compression of the left iliac vein by the right iliac artery can lead to clinical manifestations of lower limb outflow obstruction and precipitate deep venous thrombosis (DVT). Endovascular intervention of the iliac arteries is a common procedure and is frequently done without any incidence of MTS. Although rare, it is important to recognise that the implantation of a device in the iliac arteries can precipitate iatrogenic MTS while also complicating the treatment of underlying venous pathology.1–4 With an increase in iliac artery interventions and the recognition of the contribution of iliac veins in lower limb pathology, clinicians need to be aware of the implications of treating venous disease. We present a case of the treatment of a long-standing iliac vein occlusion with an overlying stent in the iliac artery.
Case Report
A 60-year-old man presented to our tertiary referral centre for review of post-thrombotic syndrome in his left lower limb. His medical history included type 1 diabetes with neuropathy, hypertension and coronary artery bypass surgery 4 years before this presentation. His presenting symptoms were of heaviness, pain and fatigue in his left leg with recurrent bouts of venous eczema. These symptoms had developed 6 years earlier after the patient developed DVT in the context of a right common iliac artery mycotic aneurysm. The patient presented to an external hospital, where the aneurysm was initially thought to be inflammatory in nature and he was given a course of steroid management. Infective symptoms and positive blood cultures led to a diagnosis of mycotic disease. Due to the patient’s emergent presentation, the aneurysm was treated with a covered stent (V12 8 × 32 mm, Atrium Medical) and a prolonged course of antibiotics. After no further degeneration was noted during follow-up, the decision was made to not perform further reconstructive surgery. However, the patient developed a left-sided iliofemoral DVT – presumed secondary to compression by the aneurysm – that was treated conservatively via anticoagulation with a vitamin K antagonist and compression stockings.
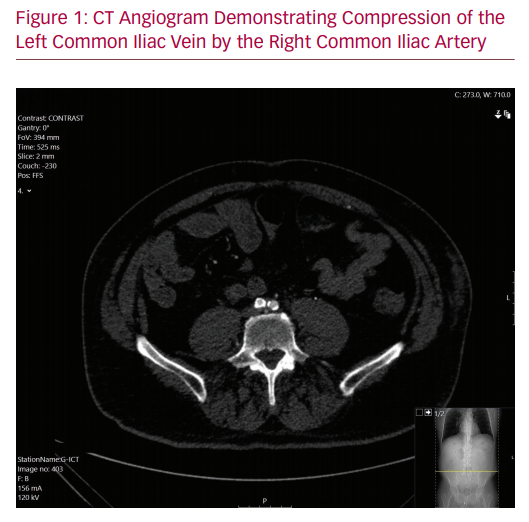
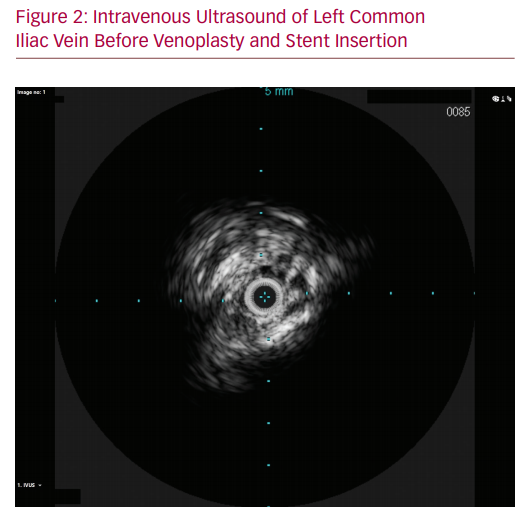
As his symptoms were severely affecting his quality of life, the patient underwent diagnostic investigations to determine his eligibility for surgical management of his disease. A venous duplex ultrasound demonstrated patent though incompetent deep veins in the left leg with incompetence of the superficial system. A formal venogram with intravascular ultrasound (IVUS) showed an occluded left common iliac venous system. The left external iliac vein was reconstituted via the left internal iliac vein with acceptable inflow. Compression of the left iliac vein at the level of the right iliac stent was noted. A CT angiogram demonstrated effacement of the iliac vein deep to the patent iliac artery stent (Figure 1). It is likely that the initial precipitating event was the arterial aneurysm. However, after the aneurysm sac involuted, the stent was causing residual compression of the vein. This was confirmed with intraoperative IVUS findings.
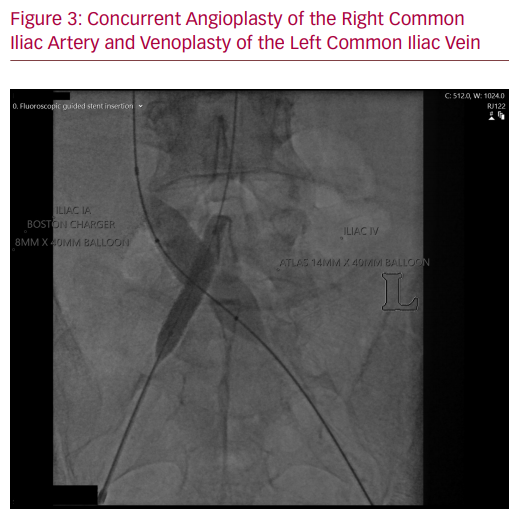
The patient’s case was discussed in a multidisciplinary venous forum consisting of vascular surgeons, interventional radiologists and haematologists. The decision was to treat the iliac lesion with venoplasty and endovenous stenting. To ensure patency of the arterial stent while the venous system was being treated, it was suggested that an intra-arterial balloon be inflated while venoplasty was performed with the high-pressure balloon.
CT Angiogram Demonstrating Compression
Intravenous Ultrasound of Left Common Iliac Vein
Discussion
Case reports have increasingly drawn attention to the role of medical implants as a possible causative mechanism for May–Thurner syndrome. Iliac artery stenting for occlusive disease has been shown previously to precipitate iliac vein compression and induce iatrogenic MTS, as has spinal fusion surgery and endovascular repair of aortic aneurysms.3–9 In cases of arterial stenting, reports describe episodes of acute DVT in the immediate post-stenting period. Compression of the vein by the newly implanted stent, along with other factors in the stenting process, such as immobility, peri-procedural cessation of anticoagulants and manual compression of the arterial puncture site, may contribute to DVT development. The management algorithm for these cases has consisted of pharmacomechanical thrombolysis, diagnostic confirmation of the compression by angiography and IVUS, venous stenting and a period of postoperative anticoagulation. The reported outcomes have been acceptable with resolution of symptoms and acceptable stent patency. No previous case report has described protecting the arterial stent during high pressure venoplasty.
The culprit for the DVT and subsequent post-thrombotic syndrome in this case was likely to be the original mycotic aneurysm (as opposed to the placement of the arterial stent) because the DVT arose before the stent placement. However, the presence of the stent meant that adjunctive manoeuvres were needed during the venous stenting to ensure there were no arterial sequelae. The high pressures required of venoplasty balloons to disrupt the fibrotic venous scarring are sufficient to cause structural failure in an arterial stent. We ensured arterial stent patency via simultaneous inflation of arterial and venous balloons to ensure there was no impingement on the arterial stent. Arterial access was gained only after the venous lesion had been crossed to reduce the risk of an unnecessary arterial puncture.
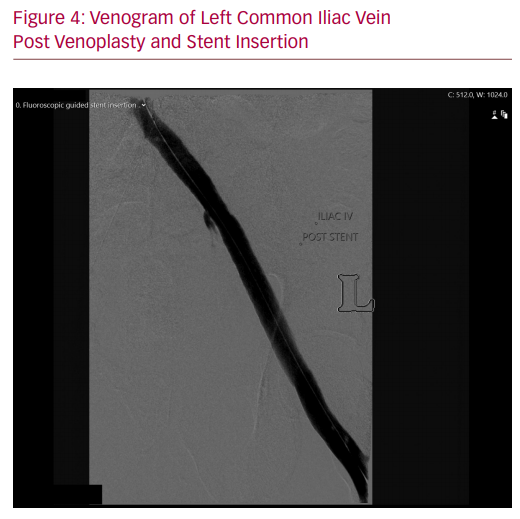
Venography and IVUS were employed in tandem to ensure accurate stent deployment. Contrast venography alone is poor at demonstrating lesions in the iliac veins, as antero-posterior compression and consequent vein effacement can demonstrate a seemingly patent vessel.10 IVUS is therefore integral in identifying the MTS compression point and ensuring the cranial extent of the stent is placed across this point without projecting excessively into the inferior vena cava. IVUS is especially advantageous in cases of iatrogenic MTS where the crossing point of the echogenic stent is easily identified.
Intravenous Ultrasound of Left Common Iliac Vein
Conclusion
The use of iliac intervention for arterial disease is rapidly increasing.11 This factor, along with the burgeoning recognition of the role iliac vein compression plays in lower limb symptoms, will result in iatrogenic MTS being encountered more frequently. Clinicians need to be alert for the potential role that stents and other medical implants have in precipitating MTS and they must develop a strategy to ensure adequate treatment of the venous system without disrupting any arterial stents. In these cases, a ‘buddy’ balloon in the arterial stent – as shown in this case report – is a prudent way of ensuring that the risks of a disrupted arterial stent are reduced.
- Jeniann AY, Hadley JB, Kuwayama DP. Atypical May–Thurner syndrome caused by endovascular aortic aneurysm repair. J Vasc Surg Cases Innov Tech 2020;6:397–400.
Crossref| PubMed - Xu F, Tian Z, Huang X, et al. A case report of May–Thurner syndrome induced by anterior lumbar disc herniation: novel treatment with radiofrequency thermocoagulation. Medicine (Baltimore) 2019;98:e17706.
Crossref| PubMed - Reddy D, Mikhael MM, Shapiro GS, et al. Extensive deep venous thrombosis resulting from anterior lumbar spine surgery in a patient with iliac vein compression syndrome: a case report and literature review. Global Spine J 2015;5:e22–7.
Crossref| PubMed - Pandit AS, Hayes M, Guiney-Borgelt S, et al. Iatrogenic May–Thurner syndrome after EVAR. Ann Vasc Surg 2014;28:e17–20.
Crossref| PubMed - Rosen E, Groben L, George JC. Rare case of bilateral common iliac vein compression by arterial stents and calcification. Vascular Disease Management 2012;9:e172–4.
- Young L, Kwon J, Arosemena M, et al. Symptomatic compression of right iliac vein after right iliac artery stent placement. J Vasc Surg Venous Lymphat Disord 2017;5:735–8.
Crossref| PubMed - Chan HY, Choke ET, Tang TY, et al. A rare cause of May–Thurner syndrome postarterial intervention. Journal of Clinical Interventional Radiology 2019;3:180–4.
Crossref - Hermany PL, Badheka AO, Mena-Hurtado CI, et al. A unique case of May–Thurner syndrome: extrinsic compression of the common iliac vein after iliac artery stenting. JACC Cardiovasc Interv 2016;9:e39–41.
Crossref| PubMed - Rachaiah JM, Goyal V, Nagesh CM, et al. An interesting case of iatrogenic May–Thurner like syndrome. International Journal of Medical Research & Health Sciences 2016;5:83–6.
- McLafferty RB. The role of intravascular ultrasound in venous thromboembolism. Semin Intervent Radiol 2012;29:10–5.
Crossref| PubMed - Upchurch GR, Dimick JB, Wainess RM, et al. Diffusion of new technology in health care: the case of aorto-iliac occlusive disease. Surgery 2004;136:812–8.
Crossref| PubMed
