Raleene Gatmaitan, Keagan Werner-Gibbings, Morad Sallam, Rachel Bell, Panos Gkoutzios
|
Abstract Splenic artery aneurysms (SAA) are a rare and life-threatening pathology. Ruptured SAA has a mortality rate of up to 25%, with increased rates of rupture in pregnancy, pseudoaneurysm, liver transplantation, portal hypertension, symptomatic SAA and diameter >2 cm. Management of SAA in pregnant women is poorly described in the literature, making treatment of these patients difficult. Furthermore, careful consideration of complications for both the mother and the foetus need to be taken into account. This case report demonstrates that conservative management with monthly surveillance MRI can be used as viable treatment option of an asymptomatic 17 mm splenic artery aneurysm in a pregnant woman. Keywords: Splenic artery aneurysm, pregnancy, peripartum, conservative, surveillance, MRI Disclosure: MS is on the Vascular & Endovascular Review editorial board; this did not influence peer review. All other authors have no conflicts of interest to declare. Received: Accepted: Published online: Correspondence Details: Keagan Werner-Gibbings, Nepean Hospital, Derby St, Kingswood, NSW 2747, Australia. E: keagan.werner-gibbings@health.nsw.gov.au Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly. |
Splenic artery aneurysms (SAA) are the most frequently encountered of the visceral aneurysms, with incidence rates up to 1% reported in the normal population.1 The most devastating complication of SAA is rupture, an event conferring mortality rates of approximately 25%. SAA is an especially concerning pathology in pregnant patients. Haemodynamic fluctuation and reduced connective tissue tensile strength during pregnancy have been theorised to contribute to the increased risk of SAA rupture, which is especially prevalent in the third trimester.2,3 Furthermore, mortality due to rupture is higher in pregnancy with maternal mortality and foetal mortality rates of 20–75% and 15–95%, respectively.4,5 The thresholds for intervening in SAA in the general population are relatively well defined: size >2 cm, symptomatic, rapid growth or liver transplantation.6 However, when an unruptured SAA is encountered in pregnancy the indications for intervention are more opaque, especially for small aneurysms. We present a case of a patient with a small splenic aneurysm that was managed conservatively over the course of her pregnancy. Informed written consent regarding case report and images has been provided by the patient.
Case Report
A 39-year-old woman presented to the vascular surgery outpatient department of our tertiary referral hospital for review of a splenic artery aneurysm in the context of being 20 weeks pregnant. Her past medical history was notable only for hypothyroidism. She had been reviewed 2 years earlier by our vascular service, having been referred from the general surgical unit, where work-up for acalculous cholecystitis had demonstrated a 17 mm splenic artery aneurysm on magnetic resonance cholangiopancreatography. At that time, a multidisciplinary team meeting consisting of vascular surgeons, general surgeons and interventional radiologists had concluded that conservative management was appropriate, due to the sub-threshold diameter and the tortuosity of the splenic artery making any spleen preserving endovascular intervention technically difficult. The patient was counselled at this time regarding the increased risks of rupture during pregnancy and the preference of the team to pre-emptively intervene on her SAA if future pregnancies were planned. She confirmed that she had no plans for any further children at that time. A 6-month follow-up scan and review confirmed stable sac size with no changes in morphology.
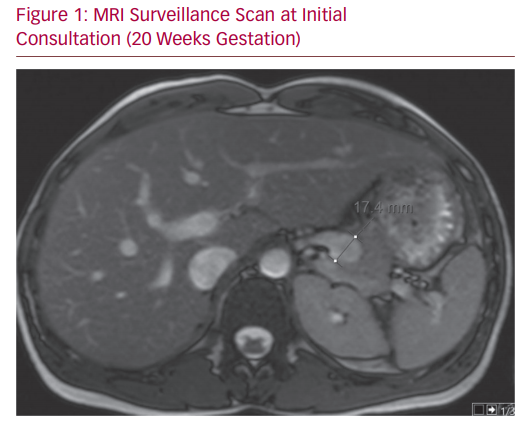
Unfortunately, the patient was lost to follow-up at this stage. Her next contact with the service came after referral from her primary care clinician, having unexpectedly become pregnant. At the time of review, she was 20 weeks pregnant and progressing normally. She had no abdominal pain or discomfort. Her examination demonstrated no abnormality. A non-contrast MRI was performed at this time which demonstrated a consistently stable SAA (Figure 1). The patient was well engaged in her health choices. A thorough discussion was undertaken concerning risks and benefits of the possible treatment pathways: conservative, endovascular and open. Well counselled about the risks, the patient expressed a strong desire to pursue a course of conservative management for her aneurysm through the course of her pregnancy. Her decision was guided by concerns regarding contrast, radiation and anaesthesia, in addition to the risks of asplenism on the foetus.
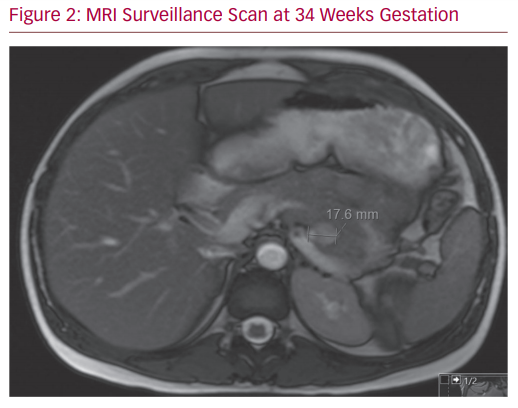
Her case was reviewed again by the multidisciplinary team. Comprehensive literature review provided minimal guidance on the case. Given the stable nature of the SAA and the patient’s wishes, a treatment plan of vigilant surveillance was enacted. Blood pressure control was optimised and the patient was referred to an obstetrician specialising in high-risk pregnancy. Serial monthly MRIs were undertaken, which demonstrated a stable SAA in both size and morphology (Figure 2). The patient’s pregnancy progressed without issue and her progress foetal ultrasound scans were reassuring. She delivered at 40 weeks with no perinatal complications. She has been booked for endovascular treatment of her SAA.
MRI Surveillance Scan at Initial Consultation
A large body of evidence describes the natural history and management of SAA in the general population. SAA seems to be more prevalent in pregnancy, with half of all ruptures occurring in this population.7 When rupture during pregnancy occurs, it is most often in the third trimester (60%), with a lesser proportion occurring during the second trimester, at birth or puerperium.8 Commonly accepted practice dictates treatment of SAA at any size in those who anticipate becoming pregnant. However the bulk of evidence pertaining to the treatment of SAA in pregnancy describes cases that are ruptured on presentation.
The appropriate management of patients with asymptomatic SAA who present already pregnant is poorly described; only isolated case reports have addressed this issue, all with differing treatment strategies.9 Laparoscopic resection of the SAA has been successfully performed in these cases with few complications. However, great care must be made with regards to the gravid uterus in operative technique.2,5 Furthermore, a single case of embolisation of the splenic artery has been reported on a 13 mm SAA in the third trimester. Although technically successful, the patient went on to develop a splenic abscess three weeks later.10 Only one case report describes conservative management of a SAA found incidentally at 25 weeks gestation. The patient went on to deliver a healthy baby at 34 weeks with elective caesarean section and post-partum embolisation.11
This lack of published literature made the treatment of our patient, who firmly opted for a conservative approach, difficult. Our decision to opt for serial surveillance was made easier due to the presence of pre-partum imaging, with which on-going comparison surveillance could be made. The availability of this baseline scan allowed assessment of size progression and morphological variation over the course of the pregnancy, factors which would have precipitated urgent treatment. This is a major advantage when compared to cases of SAA that have been identified when the patient was already pregnant. While consistency on serial imaging may have been comforting to infer SAA stability, there are no data to confirm that this is indicative of a reduced rupture risk. Our surveillance protocol was based on the premise that a stable SAA of size <20 mm was less likely to rupture than one in which changes were noted. The appropriateness of employing a size threshold as an indication for intervention is conjecture. A size of 20 mm is commonly used as the cut-off for treatment in the non-pregnant population. This absolute size indication may not hold for the pregnant cohort, where half of ruptured aneurysms have been reported as being below this diameter. Indeed, aneurysms as small as 5 mm have presented ruptured, prompting suggestions that intervention on SAA should occur in pregnancy at any size.4
We employed serial non-contrast MRI to monitor aneurysm size and morphology. MRI confers the obvious advantage over CT scanning of not employing radiation to obtain images and is safe in pregnancy. This is especially of benefit in this described case where repeated scans were necessary. MRI is also superior to ultrasound for imaging in these cases as it provides objective, topographical imaging and is less operator dependent. This is especially relevant in the latter stages of pregnancy, where the gravid uterus hampers adequate visualisation of the splenic artery anatomy. While guidelines have suggested gadolinium is likely to be safe in pregnancy, as minimal gadolinium crosses the placenta, we found non-contrast imaging was sufficient to visualise the SAA.12
If intervention is decided upon, method and timing are important considerations. Endovascular repair is the treatment of choice for anatomically amenable SAA: those with simple morphology, not immediately adjacent to the splenic hilum. Endovascular techniques confer the attendant risks of ionising radiation on the developing foetus. Radiation exposure increases the likelihood of embryo non-implantation, foetal abnormalities and childhood cancer.13 Methods to reduce radiation exposure to the foetus, such as targeted foetal shielding, low-dose fluoroscopy and minimal screening time, can assist in minimising risks.14 Furthermore, delaying the procedure as long as feasible can reduce the risk of side effects as evidence suggests risks from radiological procedures are greatest before 15 weeks of age. These risks taper as pregnancy progresses, such that the risks of intrauterine radiation after 26 weeks of gestation are similar to that of a newborn.15 This reduction in the radiation risk as the pregnancy progresses needs to be weighed against the increased risks of SAA rupture, the majority occurring in the late second or third trimester. Thus it seems that prompt treatment after 26 weeks of gestation is the ideal therapeutic window if endovascular intervention is to be undertaken.
Laparoscopic treatment for SAA unable to be treated endovascularly takes the form of splenic artery ligation and splenectomy, necessitating post-operative coverage for encapsulated bacteria. Preservation of the spleen and its immune function should be considered for SAA requiring intervention far from the splenic hilum. Laparotomy with resection of the SAA and anastomosis of the proximal and distal splenic artery with successful preservation of the spleen in a non-pregnant woman has been described.16 If laparoscopic or open intervention is required, the timing of treatment needs to consider the risks to embryogenesis of operating early, and technical difficulties with open and laparoscopic surgery posed by the gravid uterus in the third trimester. It is suggested that the second trimester is the most suitable for laparoscopic treatment.2
This case demonstrates that, adequately monitored, conservative management may be used as a treatment strategy in selected pregnant patients with known SAA. If a policy of conservative management is employed, multidisciplinary input is imperative. The patient requires adequate information to make informed decisions for her own welfare and that of her child. It is especially important to alert the patient to the symptoms that may manifest in the case of rupture and ensure there is a low threshold for attending urgent care. Early delivery has not yet been demonstrated to improve outcomes, but would seem prudent to minimise SAA rupture risk, as would aggressive management of hypertension. Serial surveillance with non-contrast MRI, where available, is appropriate.
- Stanley JC, Fry WJ. Pathogenesis and clinical significance of splenic artery aneurysms. Surgery 1974;76:907–9.
PubMed - Samamé J, Kaul A, Garza U, et al. Laparoscopic aneurysm resection and splenectomy for splenic artery aneurysm in the third trimester of pregnancy. Surg Endosc 2013;27:2988–91.
Crossref| PubMed - Trastek VF, Pairolero PC, Joyce JW, et al. Splenic artery aneurysms. Surgery 1982;91:694–9.
PubMed - Ha JF, Phillips M, Faulkner K. Splenic artery aneurysm rupture in pregnancy. Eur J Obstet Gynecol Reprod Biol 2009;146:133–7.
Crossref| PubMed - Lang W, Strobel D, Beinder E, et al. Surgery of a splenic artery aneurysm during pregnancy. Eur J Obstet Gynecol Reprod Biol 2002;102:215–6.
Crossref| PubMed - Nanez L, Knowles M, Modrall JG, et al. Ruptured splenic artery aneurysms are exceedingly rare in pregnant women. J Vasc Surg 2014;60:1520–3.
Crossref| PubMed - Holdsworth RJ, Gunn A. Ruptured splenic artery aneurysm in pregnancy. A review. Br J Obstet Gynaecol 1992;99:595–7.
Crossref| PubMed - Barrett JM, Van JH, Boehm FH. Pregnancy-related rupture of arterial aneurysms. Obstet Gynecol Surv 1982;37:557–66.
Crossref| PubMed - Sadat U, Dar O, Walsh S, Varty K. Splenic artery aneurysms in pregnancy–a systematic review. Int J Surg 2008;6:261–5.
Crossref| PubMed - Parrish J, Maxwell C, Beecroft JR. Splenic artery aneurysm in pregnancy. J Obstet Gynaecol Can 2015;37:816–8.
Crossref| PubMed - Wiener Y, Tomashev R, Neeman O, et al. Splenic artery aneurysms during pregnancy: an obstetric nightmare. Eur J Obstet Gynecol Reprod Biol 2019;237:121–5.
Crossref| PubMed - Garcia-Bournissen F, Shrim A, Koren G. Safety of gadolinium during pregnancy. Can Fam Physician 2006;52:309–10.
PubMed - Wang PI, Chong ST, Kielar AZ, et al. Imaging of pregnant and lactating patients: part 1, evidence-based review and recommendations. AJR Am J Roentgenol 2012;198:778–84.
Crossref| PubMed - Thabet A, Kalva SP, Liu B, et al. Interventional radiology in pregnancy complications: indications, technique, and methods for minimizing radiation exposure. Radiographics 2012;32:255–74.
Crossref| PubMed - Centers for Disease Control and Prevention. Radiation and pregnancy: a fact sheet for the public. Washington DC: CDC, 2011. https://emergency.cdc.gov/radiation/pdf/prenatal.pdf (accessed 1 November 2020).
- Aday U, Bozdag E, Gündes¸ E, et al. Spleen-preserving surgery in splenic artery aneurysm. Case Rep Surg 2017;2017:8716962.
Crossref| PubMed
