Thuy-My Nguyen, Saissan Rajendran, Kilian GM Brown, Prakash Saha, Raffi Qasabian
|
Abstract While the endovascular approach has been the treatment of choice for abdominal aortic aneurysm (AAA) repair in the modern era, open AAA repair remains a treatment option and may have a resurgence after the recent release of draft guidelines from the National Institute for Health and Care Excellence (NICE). Incisional hernia is a common long-term complication of open AAA repair and causes significant patient morbidity. As the number of patients undergoing open AAA repair increases, it is imperative that vascular surgeons are aware of and aim to reduce the complications associated with open surgery. This review article summarises current evidence, highlighting the risk factors for incisional hernia and the modern surgical techniques that can prevent complications. Keywords: Abdominal aortic aneurysm, incisional hernia, laparotomy, National Institute for Health and Care Excellence
Disclosure: PS is on the Vascular & Endovascular Review editorial board; this did not influence peer review. All other authors have no conflicts of interest to declare.
Received: Accepted: Published online:
Correspondence Details: Saissan Rajendran, Department of Vascular Surgery, Royal Prince Alfred Hospital, PO Box M157, Missenden Road, NSW 2050, Australia. E: saissanrajendran@hotmail.com
Open Access: This work is open access under the CC-BY-NC 4.0 License which allows users to copy, redistribute and make derivative works for non-commercial purposes, provided the original work is cited correctly.
|
Abdominal aortic surgery has seen a significant shift over the past two decades, with increasing use of endovascular techniques compared with open surgery. In contemporary practice, an endovascular approach to aortic aneurysm repair is used in more than two-thirds of elective cases and now represents the treatment of choice in the emergency setting for ruptured aneurysms if anatomically suitable.1 Despite this, a draft National Institute for Health and Care Excellence (NICE) guideline for the diagnosis and management of abdominal aortic aneurysms, released in May 2018, has recommended open repair rather than endovascular aortic repair (EVAR) for unruptured infrarenal abdominal aortic aneurysms (AAA) on the basis of cost-effectiveness and long-term outcomes. It also recommended that EVAR should not be offered to patients with unruptured infrarenal AAAs who were not considered suitable for open AAA repair because of medical comorbidity. Open repair was also recommended as the choice for repair of ruptured aneurysm in men under 70 years of age or for people with complex aneurysms.2
Although these draft guidelines have generated controversy among the international vascular surgery community and are currently being debated, it is a strong possibility that they may be implemented in the UK. Therefore, this may increase the numbers of patients having an open AAA repair which will inevitably cause a rise in specific complications from this open procedure.
One of the most common long-term complications of open AAA repair is incisional hernia. Rates for this complication are reported to be as high as 38% and it is symptomatic in more than 80% of patients.3,4 Symptoms include abdominal pain and discomfort and it can lead to life-threatening complications including bowel strangulation, intestinal obstruction and/or perforation. In addition, patients with incisional hernias report significantly lower mean scores in physical functioning, cosmetic and body image scores when compared with patients without hernias.4 Repair of an incisional hernia is required in about 10% of patients.5,6 This article reviews the risk factors for incisional hernias in patients who undergo open repair AAA and it will consider the surgical techniques that vascular surgeons could consider at the time of surgery that could mitigate the risk of an incisional hernia after surgery.
Risk Factors and Pathophysiology
AAA is an independent risk factor for incisional hernia after laparotomy. A systematic review in patients who underwent open AAA repair compared with patients undergoing laparotomy for aortoiliac occlusive disease (AOD) has reported an approximate threefold increase in risk for both inguinal and postoperative incisional hernia (OR 2.85; 95% CI [1.71–4.77]; p<0.0001 and OR 2.79; 95% CI [1.33–4.13]; p<0.0001, respectively).7
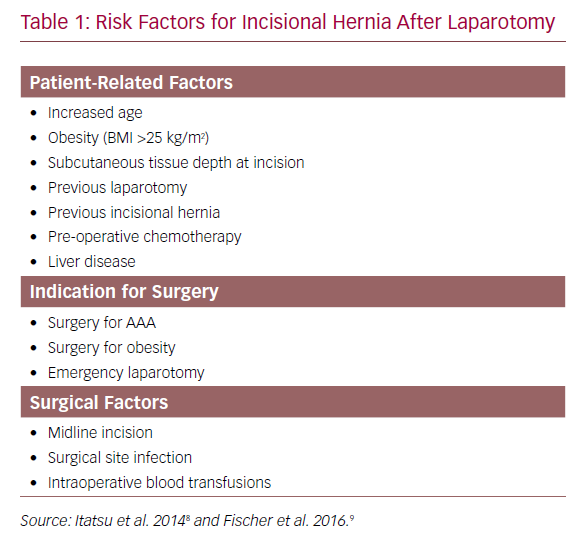
These findings were supported with data from the Danish Vascular Registry that showed AAA to be an independent risk factor for incisional hernia (HR 1.58; 95% CI [1.06–2.35]; p=0.024) when adjusted for age, American Society of Anesthesiologists (ASA) score and BMI >25 kg/m2. Although the cumulative risk of incisional hernia repair was the same in AAA and AOD by 5 years, patients with AAA had a 1.6-fold higher cumulative risk of incisional hernia repair compared with those treated for AOD (p=0.08).6 These findings suggest that while early incisional hernias may be secondary to technical failures of wound closure or wound dehiscence, incisional hernias that develop in the longer term after open AAA repair may be associated with factors involved in aneurysmal degeneration. The precise mechanism for higher rates of incisional hernia in open AAA repair is likely to be multifactorial. These can be classified into patient-related risk factors and surgery-related factors and have been listed in Table 1.8,9
Mechanism of Incisional Hernia Development
Both AAAs and abdominal wall hernias share common pathophysiological mechanisms including increased collagen breakdown caused by a protease/antiprotease imbalance. Elastin interposed between the smooth muscle cells of the tunica media and longitudinally arranged collagen in the tunica adventitia allow the aorta its structural elastic properties and strength. Collagen is the primary structural element of the adventitia and helps prevent expansion of the arterial wall beyond physiological limits during systole.10 Elastin and collagen metabolism is regulated by the aortic wall’s extracellular matrix (ECM). Imbalance between matrix metalloproteinases (MMPs) and tissue inhibitors of metalloproteinases (TIMPs) are thought to impair normal physiological aortic wall remodelling leading to aneurysm development. Several MMPs, including MMP2 and MMP9, have been implicated as the dominating proteolytic enzymes for inciting and propagating aneurysm development.11 This process is also implicated in the pathological formation of abdominal wall and inguinal hernias. Type 1 collagen is highly cross-linked and provides the abdominal fascia with its mechanical strength while type 3 collagen is less abundant, less cross-linked and offers less tensile strength to tissues. Type 3 collagen is eventually replaced with type 1 collagen during the stages of wound healing and remodelling. Similar to AAAs, the altered ratio of type 1 to type 3 collagen ratio may be a result of increased collagen metabolism caused by dysfunctional ECM activity. Studies of inguinal hernias have found an overexpression of degrading MMP2 and MMP9, as well as increased MMP1 and MMP13 activity in recurrent inguinal hernias.11,12
Prevention
Guidelines from the European Hernia Society on the closure of abdominal wall incisions recommend non-midline laparotomy incisions where possible.13 A systematic review comparing midline, transverse and paramedian incisions concluded that non-midline incisions were associated with a significantly lower rate of incisional hernias compared with those on the midline for both transverse (RR 1.77; 95% CI [1.09–2.87]) and paramedian incisions (RR 3.41; 95% CI [1.02–11.45]).14 This is in contrast to a previous study that showed no difference in the rate of incisional hernias between transverse abdominal incision compared with midline laparotomy incisions for elective infrarenal aortic reconstruction.15 However, this study included both AAA and AOD patients. In a randomised controlled trial (RCT) of patients undergoing midline versus transverse incision for open AAA repair, a significantly lower risk of incisional hernia was demonstrated if a transverse incision was used.16 Despite this, not all AAAs can be repaired with a transverse incision due to the difficulty in access for either proximal or distal control. If the anatomy is favourable, however, a transverse incision should be considered to reduce the risk of incisional hernias.
Suture Technique
The European Hernia Society recommends using slowly absorbable suture material rather than non-absorbable sutures for continuous closure of midline abdominal wall incisions.13 These recommendations are based on results of systematic reviews.17,18,19 Although there is no difference in incisional hernia rate between the two suture types, there is an increased incidence of suture sinus formation and wound pain when non-absorbable sutures are used to close the midline fascia.18,19
The beneficial effect of a high suture length to wound length (SL:WL) ratio of at least 4:1 on reducing the incidence of incisional hernias in midline laparotomy wounds is well reported.20 In patients undergoing open AAA repair, the development of an incisional hernia is associated with a longer incision, longer operating time, and a SL:WL ratio of less than 4:1 at the time of index surgery.21
Laparotomy closure has been classically taught with ‘long stitches’ placed at least 10 mm away from the wound edge. Following contrary evidence from experimental studies, a short-stitch technique for midline laparotomy wound closure has more recently been proposed.22,23 The short-stitch technique consists of placing sutures 5–8 mm from the wound edge, 5 mm from suture to suture, including only the aponeurosis, which is thought to reduce trauma and complications of infection and excessive scarring. An RCT has suggested that there is a twofold risk of wound infection (OR 2.15; 95% CI [1.17–3.96]) and a fourfold risk of incisional hernia (OR 4.24; 95% CI [2.19–8.23]) when the long-stitch technique is used.23 These outcomes were affirmed by the Suture Techniques to reduce the Incidence of The inCisional Hernia (STITCH) trial, a multicentre RCT from the Netherlands. Patients in the experimental group underwent laparotomy closure using the small-stitch technique and reported a 1-year incisional hernia rate of 13% (compared with 21% in patients closed with the large-stitch technique) (OR 0.52; CI [0.31–0.87]; p=0.0131). The small-bite technique was, however, associated with a higher SL:WL ratio (5.0 [SD 1.5] versus 4.3 [SD 1.4]; p<0.0001) and a longer closure time (14 [SD 6] minutes versus 10 [SD 4] minutes; p<0.0001).24 Although this technique is not specific to abdominal wound closure post AAA repair, the findings may still be applicable.
- Beck AW, Sedrakyan A, Mao J, et al. Variations in abdominal aortic aneurysm care: a report from the international consortium of vascular registries clinical perspective. Circulation 2016;134:1948–58. Crossref| PubMed
- National Institute for Health and Care Excellence. Abdominal Aortic Aneurysm: Diagnosis and Management. Draft for consultation, May 2018. 2018. Available at: https://www.nice.org.uk/guidance/gid-cgwave0769/documents/short-version-of-draft-guideline (accessed 17 August 2019).
- Holland AJA, Castleden WM, Norman PE, Stacey MC. Incisional hernias are more common in aneurysmal arterial disease. Eur J Vasc Endovasc Surg 1996;12:196–200. Crossref| PubMed
- van Ramshorst GH, Eker HH, Hop WC, et al. Impact of incisional hernia on health-related quality of life and body image: a perspective cohort study. Am J Surg 2012;204:144–50. Crossref| PubMed
- Lederle FA, Freischlag LA, Kyriakides TC, et al. Long-term comparison of endovascular and open repair of abdominal aortic aneurysm. N Eng J Med 2012;21:1988–97. Crossref| PubMed
- Henriksen NA, Helgstrand F, Vogt KC, et al. Risk factors for incisional hernia repair after aortic reconstructive surgery in a nationwide study. J Vasc Surg 2013;57:1524–30. Crossref| PubMed
- Takagi H, Sugimoto M, Kato T, et al. Postoperative incision hernia in patients with abdominal aortic aneurysm and aortoiliac occlusive disease: a systematic review. Eur J Vasc Endovasc Surg 2007;33:177–181. Crossref| PubMed
- Itatsu K, Yokoyama Y, Sugawara G, et al. Incidence of and risk factors for incisional hernia after abdominal sugery. Br J Surg 2014;140:1439–47. Crossref| PubMed
- Fischer J, Masta M, Mirzabeigi M, et al. A risk model and cost analysis of incisional hernia after elective abdominal surgery based upon 12,373 cases: the case of targeted prophylactic intervention. Ann Surg 2016;263:1010–17. Crossref| PubMed
- Ross M, Pawlina W. Cardiovascular system. In: Paulina W. Histology: A Text and Atlas with Correlated Cell and Molecular Biology. 5th ed. Baltimore: Lippincott Williams & Wilkins, 2006; 364–86.
- Antoniou GA, Georgiadis GS, Antoniou SA, et al. Abdominal aortic aneurysm and abdominal wall hernia as manifestation of a connective tissue disorder. J Vasc Surg 2011;54:1175–81. Crossref| PubMed
- Klinge U, Binnebösel M, Mertens PR. Are collagens the culprits in the development of incisional and inguinal hernia disease? Hernia 2006;10:471–7. Crossref| PubMed
- Muysoms FE, Antoniou SA, Bury K, et al. European Hernia Society guidelines on the closure of abdominal wall incisions. Hernia 2015;19:1–24. Crossref| PubMed
- Birkenbach KA, Karanicolas PJ, Ammor JB, et al. Up and down or side to side? A systematic review and meta-analysis examining the impact of incision on outcomes after abdominal surgery. Am J Surg 2013;3:400–9. Crossref| PubMed
- Lord RSA, Crozier JA, Snell J, Meek AC. Transverse abdominal incisions compared with midline incisions for elective infrarenal aortic reconstruction: predisposition to incisional hernia in patients with increased intraoperative blood loss. J Vasc Surg 1994;20:27–33. Crossref| PubMed
- Fassiadis N, Roidl M, Hennig M, et al. Randomized clinical trial of vertical or transverse laparotomy for abdominal aortic aneurysm repair. Br J Surg 2005;92:1208–11. Crossref| PubMed
- Diener MK, Voss S, Jensen K, et al. Elective midline laparotomy closure: the INLINE systematic review and meta-analysis. Ann Surg 2010;251:843–56. Crossref| PubMed
- van’t Riet M, Steyerberg EW, Nellensteyn J, et al. Meta-analysis of techniques for closure of midline abdominal incision. Br J Surg 2002;89:1350–6. Crossref| PubMed
- Sajid MS, Parampalli U, Baig MK, McFall MR. A systematic review on the effectiveness of slowly-absorbable versus non-absorbable sutures for abdominal fascial closure following laparotomy. Int J Surg 2011;9:615–25. Crossref| PubMed
- Israelsson La, Jonsson T. Suture length to wound length ratio and healing of midline laparotomy incisions. Br J Surg 1993;80:1284–6. Crossref| PubMed
- Israelsson LA. Incisional hernias in patients with aortic aneurysmal disease: the importance of suture technique. Eur J Vasc Endovasc Surg 1999;17:133–5. Crossref| PubMed
- Cengiz Y, Blomquist P, Israelsson LA. Small tissue bites and wound strength: an experimental study. Arch Surg 2001;136:272–5. Crossref| PubMed
- Millbourn D, Cengiz Y, Israelsson LA. Effect of stitch length on wound complications after closure of midline incisions: a randomized controlled trial. Arch Surg 2009;144:1056–9. Crossref| PubMed
- Deerenberg EB, Hariaar JJ, Steyerberg EW, et al. Small bites verus large bites for closure of abdominal midline incisions (STITCH): a double-blind multicenter randomised controlled trial. Lancet 2015;386:1254–60. Crossref| PubMed
- Payne R, Aldwinckle J, Ward S. Meta-analysis of randomised trials comparing the use of prophylactic mesh to standard midline closure in the reduction of incisional herniae. Hernia 2017;21:843–53. Crossref| PubMed
- Indrakusuma R, Jalalzadeh H, van der Meij JE, Koelemay MJW. Prophylactic mesh reinforcement versus sutured closure to prevent incisional hernias after open abdominal aortic aneurysm repair via midline laparotomy: a systematic review and meta-analysis. Eur J Vasc Endovasc Surg 2018;56:120–8. Crossref| PubMed
- Jairam AP, Timmermans L, Eker HH, et al. Prevention of incisional hernia with prophylactic only and sublay mesh reinforcement versus primary suture only in midline laparotomies (PRIMA): 2 year follow-up of a multicentre, double-blind, randomised controlled trial. Lancet 2017;10094:567–76. Crossref| PubMed
